PharmaShots Weekly Snapshots (Jul 21, 2025 – Jul 25, 2025)
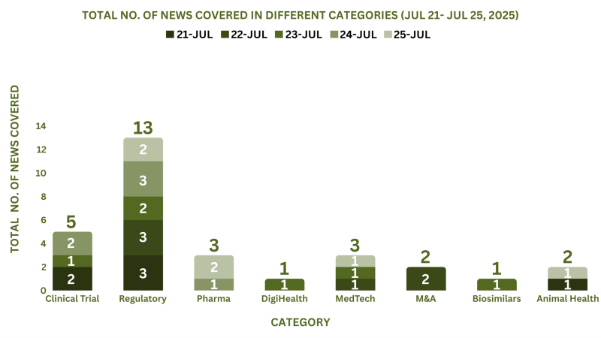
This week, PharmaShots’ news was all about the updates on clinical trials, Regulatory, Pharma, MedTech, M&A, DigiHealth, Animal Health and Biosimilars. Check out our full report below:

AstraZeneca Reports P-III (FLAURA2) Trial Findings on Tagrisso Regimen to Treat EGFRm NSCLC
Read More: AstraZeneca
Sun Pharma Reports Topline Data from P-III (INSPIRE-1 & INSPIRE-2) Trials of Ilumya (Tildrakizumab) for Active Psoriatic Arthritis
Read More: Sun Pharma
HMNC Brain Health and Spruce Biosciences Dose First Patient in P-II (TAMARIND) Trial of Tildacerfont for Major Depressive Disorder
Read More: HMNC Brain Health and Spruce Biosciences
AstraZeneca Reports P-III (PREVAIL) Trial Data of Gefurulimab for Generalised Myasthenia Gravis (gMG)
Read More: AstraZeneca
Apnimed Reports Topline P-III (LunAIRo) Trial Data of AD109 for Obstructive Sleep Apnea (OSA)
Read More: Apnimed

The EC Grants Conditional Approval to SpringWorks Therapeutics’ Ezmekly for NF1-PN
Read More: SpringWorks Therapeutics
ARS Pharmaceuticals and ALK’s EURneffy Secures the MHRA’s Approval to Treat Allergic Reactions in Children
Read More: ARS Pharmaceuticals and ALK
Bayer Reports EC’s Approval of Nubeqa for Treating Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)
Read More: Bayer
Johnson & Johnson Submits NDA to the US FDA for Icotrokinra to Treat Plaque Psoriasis (PsO)
Read More: J&J
Merck’s Keytruda Regimen Receives Health Canada’s Approval for FIGO 2014 Stage III-IVA Cervical Cancer
Read More: Merck
Bavarian Nordic Reports the Health Canada’s NDS Acceptance of CHIKV VLP to Prevent Chikungunya
Read More: Bavarian Nordic
Roche’s Itovebi Regimen Secures the EC’s Approval for PIK3CA-mutated Breast Cancer
Read More: Roche
Johnson & Johnson’s Imbruvica Receives the EC’s Approval for Previously Untreated Mantle Cell Lymphoma (MCL)
Read More: J&J
Johnson & Johnson’s Darzalex Secures the EC’s Approval for High-Risk Smouldering Multiple Myeloma
Read More: J&J
GSK Receives Health Canada’s Approval for Blenrep (Belantamab Mafodotin) Regimens to Treat R/R Multiple Myeloma
Read More: GSK
LEO Pharma’s Anzupgo (Delgocitinib) Gains the US FDA’s Approval to Treat Chronic Hand Eczema (CHE)
Read More: LEO Pharma
GSK’s Blenrep (Belantamab Mafodotin) Regimens Receive the EC’s Approval to Treat R/R Multiple Myeloma
Read More: GSK
Exelixis and Ipsen Report the EC’s Approval of Cabometyx (Cabozantinib) to Treat Advanced Neuroendocrine Tumors (NETs)
Read More: Exelixis and Ipsen

Kling Bio Joins Forces with Sanofi to Identify Neutralizing Antibodies
Read More: Kling Bio and Sanofi
Matchpoint Therapeutics Collaborates with Novartis to Develop Oral Covalent Inhibitors for Various Inflammatory Diseases
Read More: Matchpoint Therapeutics and Novartis
Eli Lilly Signs a ~856M Deal with Gate Bioscience to Develop Molecular Gate Therapeutics
Read More: Eli Lilly and Gate Bioscience

Medtronic Reports the European CE Mark of MiniMed 780G System for Automated Insulin Delivery
Read More: Medtronic
Roche’ Elecsys pTau181 Test Receives the European CE Mark Approval to Rule Out Alzheimer’s Disease
Read More: Roche
Minnesota Medical Technologies Receives the US FDA’s 510(k) Clearance for StaySure to Manage Fecal Incontinence
Read More: Minnesota Medical Technologies

Sanofi to Acquire Vicebio for ~$1.6B
Read More: Sanofi and Vicebio
Concentra Biosciences Enters a Merger Agreement to Acquire iTeos Therapeutics
Read More: Concentra Biosciences and iTeos Therapeutics

The EC Approves Fresenius’ Conexxence and Bomyntra (Biosimilars, Prolia & Xgeva)
Read More: Fresenius

Slingshot AI launches Ash for Personalized Mental Health Support
Read More: Slingshot AI

Merck Animal Health Reports the US FDA’s Approval of Exzolt for Northern Fowl Mites
Read More: Merck Animal Health
Elanco’s Zenrelia Secures the EC’s Approval to Treat Allergy and Atopic Dermatitis in Dogs
Read More: Elanco
Related Post: PharmaShots Weekly Snapshots (Jun 14, 2025 – Jul 18, 2025)
