PharmaShots Weekly Snapshots (Jun 23, 2025 – Jun 27, 2025)
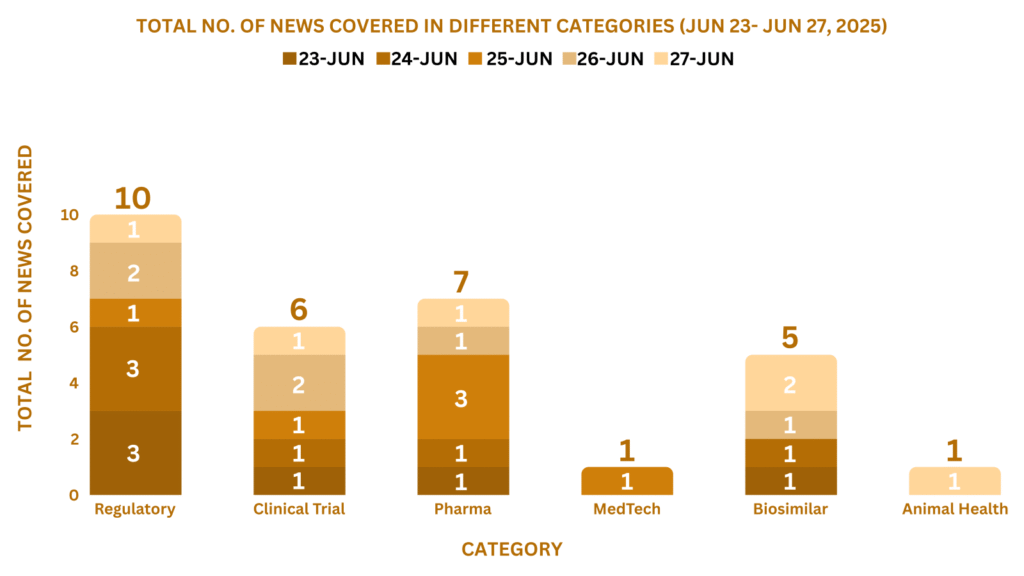
This week, PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Biosimilar, and Animal Health. Check out our full report below:

Teva Reports the Data from the P-IV (PEARL) Study of Ajovy (fremanezumab) for Chronic and Episodic Migraine Prevention
Read More: Teva
Merck Reports the Data from P-III (HYPERION) Study of WINREVAIR (sotatercept-csrk) in Adults with Recently Diagnosed PAH
Read More: Merck
Verastem Oncology Reports First Patient Dosing with VS-7375 in P-I/IIa Study for KRAS G12D Advanced Solid Tumors
Read More: Verastem Oncology
Bio-Thera Solutions Reports First Patient Dosing in P-III Study Evaluating BAT8006 for Pt Resistant Ovarian Cancer
Read More: Bio-Thera Solutions
Galderma Initiates P-II Studies of Nemolizumab for Systemic Sclerosis (SSc) and Chronic Pruritus of Unknown Origin (CPUO)
Read More: Galderma
Pfizer Reports the P-III (BASIS) Study Data Evaluating HYMPAVZI to Treat Hemophilia A or B with Inhibitors
Read More: Pfizer

Madrigal Pharmaceuticals’ Rezdiffra Receives the CHMP’s Positive Opinion for MASH with Liver Fibrosis
Read More: Madrigal Pharmaceuticals
Argenx Receives the EC’s Approval for Vyvgart SC to Treat Chronic Inflammatory Demyelinating Polyneuropathy
Read More: Argenx
SpringWorks Therapeutics’ Nirogacestat Receives the CHMP’s Positive Opinion for Desmoid Tumors
Read More: SpringWorks Therapeutics
PTC Therapeutics Receives the EC’s Approval for Sephience to Treat Phenylketonuria (PKU)
Read More: PTC Therapeutics
Accord Healthcare Reports the MHRA’s Approval of Hetronifly as 1L Treatment of ES-SCLC
Read More: Accord Healthcare
DARZALEX (Daratumumab) Receives Positive CHMP Opinion for High-Risk Smouldering Multiple Myeloma
Read More: J&J
GSK Receives the US FDA’s Approval for Benlysta to Treat Active Lupus Nephritis (LN)
Read More: GSK
GSK Reports the EMA’s MAA Acceptance of Linerixibat for Cholestatic Pruritus
Read More: GSK
Daiichi Sankyo and AstraZeneca Report the US FDA Approval of Datroway for Advanced EGFR-Mutated NSCLC
Read More: Daiichi Sankyo and AstraZeneca
BeOne Receives CHMP Positive Opinion for New Brukinsa Tablet Formulation Across All Approved Indications
Read More: BeOne

Harbour BioMed Enters a ~$670M Partnership with Otsuka Pharmaceutical to Advance HBM7020 for Autoimmune Diseases
Read More: Harbour BioMed & Otsuka Pharmaceutical
Johnson & Johnson launches ACUVUE OASYS MAX 1-Day MULTIFOCAL for ASTIGMATISM Contact Lens in the US and Canada
Read More: Johnson & Johnson
Xcell Biosciences and ThermoFisher Enter Into a Joint Research Collaboration to Advance Cell Therapies
Read More: Xcell Biosciences and ThermoFisher
Royalty Pharma Purchases Revolution Medicines’ Royalty Rights of Daraxonrasib for ~2B
Read More: Royalty Pharma & Revolution Medicines
Libertas Bio Licenses Gusacitinib to Sanofi
Read More: Formation Bio & Sanofi
Gilead Sciences and Kymera Therapeutics Sign Exclusive Option and License Agreement to Develop Oral CDK2 Degraders
Read More: Gilead Sciences and Kymera Therapeutics
Vor Bio Enters a ~$4.12B Exclusive Global License Agreement with RemeGen to Develop and Commercialize Telitacicept
Read More: Vor Bio and RemeGen

Nitinotes Reports the US FDA’s IDE Approval of EndoZip System for Endoscopic Sleeve Gastroplasty
Read More: Nitinotes

Vetigenics Doses First Patient in CHECKMATE K9 Study Evaluating VGS 001 + VGS 002 in Dogs with Solid Tumors
Read More: Vetigenics

Bio-Thera Solutions Secures the CHMP’s Positive Opinion for Usymro (Biosimilar, Stelara)
Read More: Bio-Thera Solutions
Alvotech & Advanz Pharma Secure the CHMP’s Positive Opinion for AVT06 (Biosimilar, Eylea)
Read More: Alvotech & Advanz Pharma
Bio-Thera Solutions and SteinCares Broaden Collaboration to Commercialize (Biosimilar, Dupilumab) in Latin America
Read More: Bio-Thera Solutions and SteinCares
Kashiv BioSciences and Amneal Pharmaceuticals Report Topline Results from the Study of ADL-018 (Biosimilar, Xolair) for CIU/CSU
Read More: Kashiv BioSciences and Amneal Pharmaceuticals
Biocon Biologics Reports the Health Canada’s Approval of Yesafili (Biosimilar, Eylea)
Read More: Biocon Biologics
Related Post: PharmaShots Weekly Snapshots (Jun 16, 2025 – Jun 20, 2025)
