PharmaShots Weekly Snapshots (Sep 01, 2025 – Sep 05, 2025)
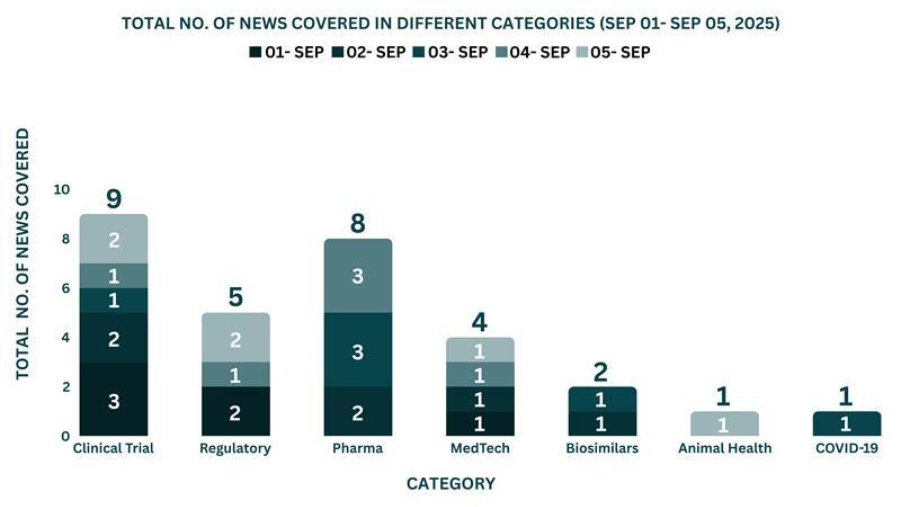
This week, PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, COVID-19, Animal Health and Biosimilars. Check out our full report below:

Merck Reports P-III (VICTOR) Trial Data of Verquvo (Vericiguat) for Chronic Heart Failure and Reduced Ejection Fraction (HFrEF)
Read More: Merck
AstraZeneca Reports P-III (BaxHTN) Trial Data on Baxdrostat for Uncontrolled or Treatment Resistant Hypertension
Read More: AstraZeneca
Zydus Reports Topline P-IIb/III (EPICS-III) Trial Data of Saroglitazar for Primary Biliary Cholangitis (PBC)
Read More: Zydus
Novo Nordisk Reports STEER Real-World Study Data on Wegovy for Obesity and Cardiovascular Disease
Read More: Novo Nordisk
Novartis Reports P-IV (V-DIFFERENCE) Trial Findings on Leqvio (Inclisiran) for Hypercholesterolemia
Read More: Novartis
Merck Reports Topline P-III (CORALreef Lipids) Trial Data of Enlicitide Decanoate to Treat Hypercholesterolemia
Read More: Merck
Ionis Reveals Topline P-III (CORE & CORE2) Trial Data of Olezarsen for Severe Hypertriglyceridemia
Read More: Ionis
Bluejay Therapeutics Reports First Patient Enrolment in P-III (AZURE-2) Trial of Brelovitug for Chronic Hepatitis D
Read More: Bluejay Therapeutics
Genentech Presents Long-Term Efficacy and Safety Data on Vabysmo for Wet Age-Related Macular Degeneration
Read More: Genentech

Sanofi’s Wayrilz (Rilzabrutinib) Receives the US FDA’s Approval for Immune Thrombocytopenia (ITP)
Read More: Sanofi
The NMPA Grants Conditional Approval to Boehringer Ingelheim’s Hernexeos for HER2-Mutant NSCLC
Read More: BI
AbbVie’s Elahere (Mirvetuximab Soravtansine) Receives the Health Canada’s Approval to Treat Platinum-Resistant Ovarian Cancer
Read More: AbbVie
The US FDA Grants Breakthrough Therapy Designation to Eli Lilly’s Olomorasib for KRAS G12C-Mutant NSCLC
Read More: Eli Lilly
Pulmovant and Roivant’s Mosliciguat Receives MHLW’s Orphan Drug Designation for Pulmonary Hypertension Associated with Interstitial Lung Disease
Read More: Pulmovant and Roivant

OMass Therapeutics Collaborates with Genentech to Develop and Commercialize Small Molecule for Inflammatory Bowel Disease
Read More: OMass Therapeutics and Genentech
IDEAYA Biosciences Partners with Servier to Develop and Commercialize Darovasertib for Uveal Melanoma (UM)
Read More: IDEAYA Biosciences and Servier
Arrowhead Enters a ~$2.2B Deal with Novartis for ARO-SNCA and TRiM-Based Therapies to Treat Neurodegenerative Diseases
Read More: Arrowhead and Novartis
Enlaza Therapeutics Collaborates with Vertex to Develop Drug Conjugates and T-Cell Engagers for Improved Conditioning and Autoimmune Diseases
Read More: Enlaza Therapeutics and Vertex
Novatim Immune Therapeutics (Keyi Pharmaceutical) Inks a ~$1.16B Licensing Deal with RADIANCE Biopharma for KY-0301 to Treat Cancer
Read More: Novatim Immune Therapeutics and RADIANCE Biopharma
Argo Biopharma Inks a ~$5.3B Deal with Novartis for Multiple Cardiovascular Assets, Expanding their Existing Partnership
Read More: Argo Biopharma and Novartis
Zenas BioPharma Enters a ~$300M Funding Agreement with Royalty Pharma to Support Obexelimab Development
Read More: Zenas BioPharma and Royalty Pharma
Biocytogen Partners with Merck KGaA to Develop Antibody-Conjugated Lipid-Based Delivery Solutions
Read More: Biocytogen and Merck KGaA

Abbott’s Navitor TAVI System Receives European CE Mark for Aortic Stenosis in Patients at Low or Intermediate-Risk for Surgery
Read More: Abbott
GE HealthCare Launches Vivid Pioneer for Enhanced Cardiac Imaging
Read More: GE HealthCare
AliveDx Reports the US FDA’s 510(k) Submission for MosaiQ AiPlex VAS Assay to Diagnose Autoimmune Vasculitis
Read More: AliveDx
Roche Reports the European CE Mark Approval of Contivue to Treat Neovascular Age-Related Macular Degeneration
Read More: Roche

Polpharma Biologics Enters into Licensing Deals with MS Pharma for Multiple Biosimilar Candidates
Read More: Polpharma Biologics and MS Pharma
Henlius and Organon Receive the US FDA’s Approval for Bildyos & Bilprevda (Biosimilars, Prolia & Xgeva)
Read More: Henlius and Organon

SignalPET Launches SignalPET 360° for Comprehensive Veterinary Radiology
Read More: SignalPET

Shionogi Reports the US FDA’s Acceptance of Ensitrelvir Post-Exposure COVID-19 Prevention
Read More: Shionogi
Related Post: PharmaShots Weekly Snapshots (Aug 25, 2025 – Aug 29, 2025)

