PharmaShots Weekly Snapshots (Aug 25, 2025 – Aug 29, 2025)
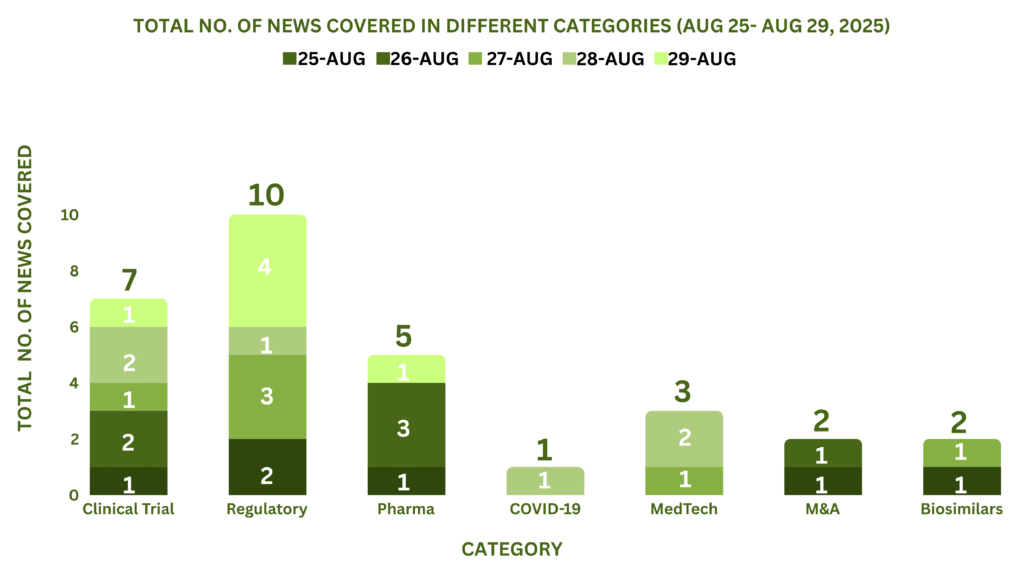
This week, PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, COVID-19, M&A and Biosimilars. Check out our full report below:

argenx Reports Topline P-III (ADAPT SERON) Trial Data of Vyvgart for AChR-Ab Seronegative Generalized Myasthenia Gravis
Read More: argenx
PDS Biotech Reports Final P-II (VERSATILE-002) Survival Data for PDS0101 + Keytruda in 1L HPV16+ Head & Neck Cancer
Read More: PDS Biotech
Merz Therapeutics Enrolls First Patients in P-III Studies Evaluating Xeomin for Migraine Prevention
Read More: Merz Therapeutics
Regeneron Reports P-III (NIMBLE) Trial Data of Cemdisiran for Generalized Myasthenia Gravis (gMG)
Read More: Regeneron
Daiichi Sankyo and Merck Dose First Patient in P-III (HERTHENA-Breast04) Trial of Patritumab Deruxtecan to Treat HR+/HER2- Breast Cancer
Read More: Daiichi Sankyo and Merck
Eli Lilly Reports Topline P-III (monarchE) Trial Data of Verzenio (Abemaciclib) to Treat Early Breast Cancer
Read More: Eli Lilly
BeOne Medicines Reports Topline P-III (BGB-11417-201) Trial Data on Sonrotoclax in R/R Mantle Cell Lymphoma (MCL)
Read More: BeOne Medicines

Daiichi Sankyo Reports the MHLW’s Approval of Enhertu for HER2 Low/Ultralow Metastatic Breast Cancer (MBC)
Read More: Daiichi Sankyo
Daiichi Sankyo’s Datroway (Datopotamab Deruxtecan) Receives the NMPA’s Approval for Unresectable or Recurrent HR+/HER2- Breast Cancer
Read More: Daiichi Sankyo
Gilead Reports the EC’s Approval of Yeytuo (Lenacapavir) for Pre-Exposure Prophylaxis (PrEP) to Prevent HIV in Individuals at Risk
Read More: Gilead
Bayer Reports the US FDA’s NDA Acceptance of Gadoquatrane for Contrast-Enhanced MRI
Read More: Bayer
BeOne Medicine’s Perioperative Tevimbra (Tislelizumab) Receives the EC’s Approval for Resectable NSCLC
Read More: BeOne Medicine
AbbVie’s Rinvoq (Upadacitinib) Receives the Health Canada Approval for Giant Cell Arteritis
Read More: AbbVie
ExCellThera Reports the EC’s Conditonal Approval of Zemcelpro for Haematological Malignancies
Read More: ExCellThera
Camurus Receives the MHRA’s Approval for Oczyesa as Maintenance Treatment of Acromegaly
Read More: Camurus
TOLREMO Therapeutics’ TT125-802 Secures 2 US FDA’s Fast-Track Designation for NSCLC with EGFR & KRAS-G12C Mutation
Read More: TOLREMO Therapeutics
Foresee Pharmaceuticals’ Camcevi ETM Receives the US FDA Approval for Advanced Prostate Cancer
Read More: Foresee Pharmaceuticals

Eisai and Biogen Report EU Launch of Leqembi (Lecanemab) to Treat Alzheimer’s Disease
Read More: Eisai and Biogen
AbbVie Acquires Gilgamesh Pharmaceuticals’ Bretisilocin to Expand Psychiatry Pipeline for ~$1.2B
Read More: AbbVie and Gilgamesh Pharmaceuticals
Royalty Pharma Signs Agreement to Acquire Interest in Amgen’s Imdelltra Royalties from BeOne Medicines for ~$950M
Read More: Royalty Pharma, Amgen, & BeOne Medicines
BioArctic and Novartis sign agreement for BrainTransporter ~$802M
Read More: BioArctic and Novartis
Replicate Bioscience Collaborates with Novo Nordisk to Develop Novel srRNA Therapies for Cardiometabolic Diseases
Read More: Replicate Bioscience and Novo Nordisk

Airiver Medical Receives the US FDA’s IDE Approval to Study Airiver Pulmonary Drug Coated Balloon for Benign Central Airway Stenosis
Read More: Airiver Medical
Abbott Receives the European CE Mark for Esprit BTK System to Treat Peripheral Artery Disease Below the Knee
Read More: Abbott
BioPorto Launches ProNephro AKI (NGAL) to Detect Acute Kidney Injury in the US
Read More: BioPorto

Terumo to Acquire OrganOx for ~$1.5B
Read More: Terumo and OrganOx
Mannkind Acquires Scpharmaceuticals to Strengthen Its Cardiometabolic and Lung Diseases Portfolio
Read More: Mannkind and Scpharmaceuticals

Bio-Thera Solutions Expands its Partnership with STADA for BAT1806 (Biosimilar, RoActemra)
Read More: Bio-Thera and STADA
Bio-Thera Secures the EC’s Approval for Usymro (Biosimilar, Stelara)
Read More: Bio-Thera

Pfizer and BioNTech’s Comirnaty Receives the US FDA Approval for Active Immunization Against COVID-19
Read More: Pfizer and BioNTech
Related Post: PharmaShots Weekly Snapshots (Aug 18, 2025 – Aug 22, 2025)

