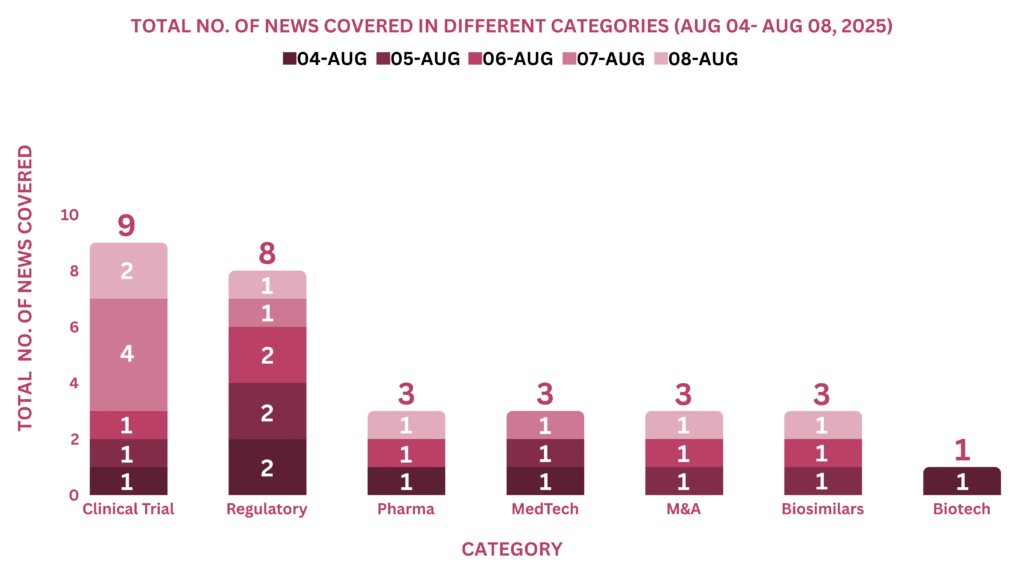
PharmaShots Weekly Snapshots (Aug 04, 2025 – Aug 08, 2025)
This week, PharmaShots’ news was all about the updates on clinical trials, Regulatory, Pharma, MedTech, Biotech, M&A and Biosimilars. Check out our full report below:

Roche Reports P-III (Portal) Trial Data on Susvimo for Neovascular Age-Related Macular Degeneration (nAMD)
Read More: Roche
Ethris Reports First Patient Dosing in P-IIa Trial of ETH47 for Asthma
Read More: Ethris
UroGen Pharma Reports P-III (ENVISION) Trial Data on Zusduri to Treat LG-IR-NMIBC
Read More: Urogen Pharma
Vertex Reports the P-II Study of VX993 in Acute Pain After Bunionectomy
Read More: Vertex
Marea Therapeutics Reports First Patient Dosing in P-IIb (TYDAL-TIMI 78) Trial of MAR001 for Atherosclerotic Cardiovascular Disease
Read More: Marea Therapeutics
Fapon Biopharma Reports First Patient Enrolment in P-I Trial of FP008 for Solid Tumors
Read More: Fapon Biopharma
Flare Therapeutics Reports First Patient Dosing in P-Ib Trial of FX-909 for Urothelial Cancer
Read More: Flare Therapeutics
Sebela Pharmaceuticals Reports P-III (TRIUMpH) Program Completion and Data on Tegoprazan for Gastroesophageal Reflux Disease (GERD)
Read More: Sebela Pharmaceuticals
Genmab Reports P-III (EPCORE FL-1) Trial Data on Epcoritamab for R/R Follicular Lymphoma
Read More: Genmab

Anbogen Therapeutics Reports the US FDA’s IND Clearance of ABT-301 for Metastatic Colorectal Cancer
Read More: Anbogen Therapeutics
The US FDA Grants Fast Track Designation to Revalesio’s RNS60 for Treating Acute Ischemic Stroke
Read More: Revalesio
BMS Reports the US FDA’s sBLA Acceptance and Priority Review of Breyanzi for R/R Marginal Zone Lymphoma (MZL)
Read More: BMS
Innovent Reports the US FDA’s IND Clearance of IBI3032 for Cardiometabolic Disorders
Read More: Innovent
Galapagos Secures the US FDA’s RMAT Designation for GLPG5101 to Treat R/R Mantle Cell Lymphoma
Read More: Galapagos
The US FDA Grants Fast Track Designation to Dizal’s Birelentinib for Treating R/R CLL/SLL
Read More: Dizal

Sanofi Enters a ~$395M Asset Purchase Agreement with Visirna Therapeutics (Arrowhead) for Plozasiran in Greater China
Read More: Sanofi and Visirna Therapeutics
Knight Therapeutics and Incyte Expand Latin America Agreement to Include Retifanlimab and Axatilimab
Read More: Knight Therapeutics and Incyte
DoveTree Medicines Enters a ~$5.99B Deal with XtalPi to Discover Novel Therapeutics Across Various Indications
Read More: DoveTree Medicines and XtalPi

Materna Medical Reports Enrolment Completion in EASE Trial of Ellora System to Reduce Pelvic Injury from Vaginal Delivery
Read More: Materna Medical
AliveDx Receives the CE Mark Approval for MosaiQ AiPlex VAS Assay to Diagnose Autoimmune Vasculitis
Read More: AliveDx
Bracco Imaging Reports the NMPA’s Approval of SonoVue for Assessment of Fallopian Tube Patency
Read More: Bracco Imaging
Roche Receives the US FDA’s 510(k) Clearance for cobas Respiratory 4-flex for Comprehensive Respiratory Pathogen Detection
Read More: Roche
TransMedics Receives FDA Conditional IDE Approval to Initiate OCS ENHANCE Heart Study
Read More: TransMedics

Alcon to Acquire STAAR Surgical Company for ~$1.5B
Read More: Alcon and STAAR Surgical
SERB Pharmaceuticals to Acquire Y-mAbs Therapeutics for ~$412M
Read More: SERB Pharmaceuticals and Y-mAbs Therapeutics
Syndicate of Global Investors to Acquire HistoSonics for $2.25B
Read More: HistoSonics

Polpharma Biologics Collaborates with Fresenius Kabi to Commercialize PB016 (Biosimilar, Entyvio)
Read More: Polpharma Biologics and Fresenius Kabi
Kashiv BioSciences and MS Pharma Sign MENA License & Supply Deal for ADL-018 (Biosimilar, Xolair)
Read More: Kashiv BioSciences and MS Pharma
Celltrion Secures the US FDA’s Approval for Avtozma IV (Biosimilar, Actemra) to Treat Cytokine Release Syndrome
Read More: Celltrion

Turbine Collaborates with Merck to Use AI-Driven Tumor Simulation for Identifying Novel Targets in Hard-to-Treat Cancers
Read More: Turbine and Merck
Related Post: PharmaShots Weekly Snapshots (Jul 28, 2025 – Aug 01, 2025)

