J.P. Morgan Special: Dealmaker 2022 – Top M&A Deal of Roche (Part 01)
Shots:
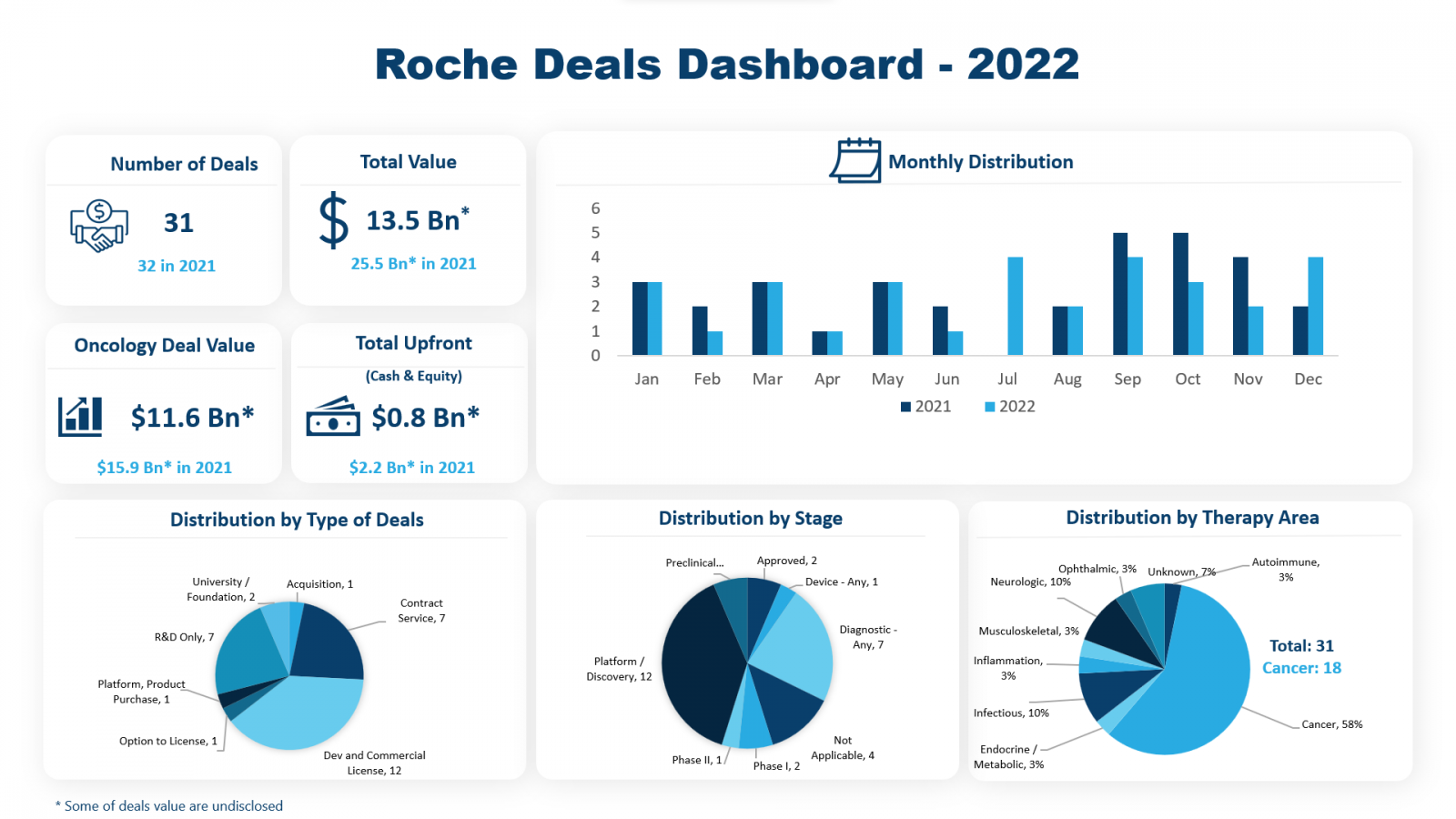
- Roche was the top dealmaker in 2022 with a completion of 31 deals with multiple pharma, biotech companies, and universities for a total deal value of $13.55B
- The highest deal was secured by Roche under its development and commercialization collaboration with Poseida for the R&D of multiple existing and novel cell therapies for the treatment of multiple myeloma, B-cell lymphomas, and other hematologic indications
- Sanofi, Eli Lilly, and BMS are also among the top dealmakers. Leveraging the data from DealForma, our team at PharmaShots has compiled insightful data for the Deal Maker of the Year
Summary
The pharmaceutical industry’s voyage through 2022 was a rollercoaster ride in what turned out to be another turbulent year. While the pharmaceutical industry was still facing the pressure of the global pandemic, the initiation of the Russia-Ukraine War possessed another significant setback to the industry, along with the regulatory pressure of the US FDA & US FTC planning to bring in “Anti-Trust Law”. Even during these tough times, Biopharma companies were able to sign a significant number of deals in the year 2022. Based on the trends of dealmaking in the healthcare sector, our team at PharmaShots has worked on the Dealmaker of the Year, 2022 by analysing the data from DealForma. Based on numbers, some of the front-runners for the dealmaker of the year were Roche (31), Sanofi (23), Eli Lilly (20), and BMS (20). Roche, which had the highest number of agreements signed in 2022, took the top spot on the list. PharmaShots presents to you a compilation of the top dealmaking highlights of Roche in the year 2022.
This year, Roche was involved in 31 deals comprising acquisitions, contract services, development & commercialization license, R&D, and product purchase among others. Roche withholds a total announced deal value of $13.55B, excluding the undisclosed deals, in the year 2022. Out of the total 31 deals, the maximum number of deals signed were under development and commercialization licensing (N=12) followed by contract services agreements (N=7) and R&D (N=7). Additionally, Roche signed 2 university partnerships along with an acquisition, an option to license, and a platform or product purchase. Roche functions under two divisions, Pharmaceutical & Diagnostics. The company aims to provide products in the therapy areas including Oncology, Immunology, Ophthalmology, Infectious Diseases, Neuroscience, and Rare Diseases.

Top Deals for Roche
Roche and Poseida Sign a Development and Commercialization Deal for CAR-T Cell Therapies to Treat Hematologic Malignancies
- Roche received the exclusive global rights for CAR-T cell therapy products including P-BCMA-ALLO1 & P-CD19CD20-ALLO1 (Tier 1 program) along with options for P-BCMACD19-ALLO1 & P-CD70-ALLO1 (Tier 2 program)
- Additionally, Roche also gained the rights to develop, manufacture & commercialize up to 6 CAR-T cell therapy products under the collaboration program along with a non-exclusive right for up to 3 cell therapy products discovered by Roche for solid tumors
- Poseida will receive $110M up front, $110M in near-term payments, milestones & other payments along with eligibility to receive up to $6B & $1.5B for the Tier 1 program, $1.1B for the Tier 2 program, $2.9B for collaboration programs & $415M for the licensed products. The company will also receive mid-single to low double-digit royalties for the Tier 1 and Tier 2 programs & low to mid-single digits royalties for licensed products
Roche and Jnana Therapeutics Sign a Development and Commercialization Agreement for Small Molecule Therapies
- Under the terms of the agreement, Jnana will receive $50M up front and is eligible for undisclosed development and regulatory milestones in addition to up to $2B in commercial milestones and tiered royalties
- Additionally, Roche received the exclusive, worldwide rights to develop and commercialize small molecule therapies for the treatment of cancer, immune-mediated, and neurological diseases
- Roche will develop these small molecule therapies by utilizing Jnana’s RAPID chemo-proteomics platform. Jnana will be responsible for all the discovery and preclinical activities whereas Roche will be responsible for all clinical development and commercial activities
Related Post: Jnana Therapeutics Entered into a Second Collaboration and License Agreement with Roche to Discover Small Molecule Drugs
Roche and Repare Therapeutics Enters into a License and Collaboration Agreement for Camonsertib
- Under the terms of the agreement, Repare will receive $125M up front and is eligible to receive up to $1.2B in clinical, regulatory, and sales milestones along with high-single-digit to high-teens royalties
- Repare granted Roche exclusive, worldwide rights to develop and commercialize Camonsertib (RP-3500) for the treatment of tumors with specific synthetic-lethal genomic alterations whereas Roche is expected to expand the development of Camonsertib into additional tumors and multiple combination studies
- If approved by the US FDA, Repare has the option of 50/50 co-development and profit share in the US whereas, if exercised, Repare will be eligible for clinical, regulatory, and sales milestones along with royalties outside of the US
Roche and Avista Enter into Collaboration to Develop AAV Gene Therapy Vectors for Ocular Diseases
- Avista will receive $7.5M up front and is eligible for up to $992.5M in development, regulatory, and commercial milestones including royalties
- Roche received the rights to develop and commercialize AVV gene therapy vectors by using the scAAVengr platform along with the right to license developed capsids and will be responsible for all preclinical, clinical, and commercialization activities
- The collaboration utilizes Avista’s scAAVengr platform technology to develop intravitreal AVV capsids matching a capsid profile defined by Roche
Related Post: Roche Collaborated with Avista to Develop AAV Gene Therapy Vectors for the Treatment of Ocular Diseases
Roche and HOOKIPA Enter into a Research Collaboration to Develop HB-700 and Novel Arenaviral Immunotherapy for KRAS Mutated Cancers
- Under the terms of the agreement, HOOKIPA will receive $25M up front and is eligible for up to $335M in development and regulatory milestones along with up to $250M in commercial milestones including tiered high single-digit to mid-teens royalties for HB-700
- If exercised, HOOKIPA will receive a $15M option exercise fee and is also eligible for up to $173M in development and regulatory milestones, and up to $160M in commercial milestones including undisclosed royalties for the UCA program
- HOOKIPA will be responsible for research and Phase Ib clinical trials of HB-700 whereas Roche will be responsible for all development and commercial activities of both therapies for the treatment of KRAS-mutated lung, colorectal, pancreatic, and other cancers
Related Post: HOOKIPA and Roche Enter into a License Agreement to Develop HB-700 and Arenaviral Immunotherapies for KRAS-Mutated Cancers
Disclaimer:
- PharmaShots has not included clinical trial agreements and older deals with only milestone payments in 2022
- Overall deal value is higher than mentioned as Roche has ~17 undisclosed deals
Related Posts: Deal Maker of the Year 2021 (Part 01)



