
PharmaShots Weekly Snapshots (October 14 – October 18, 2024)
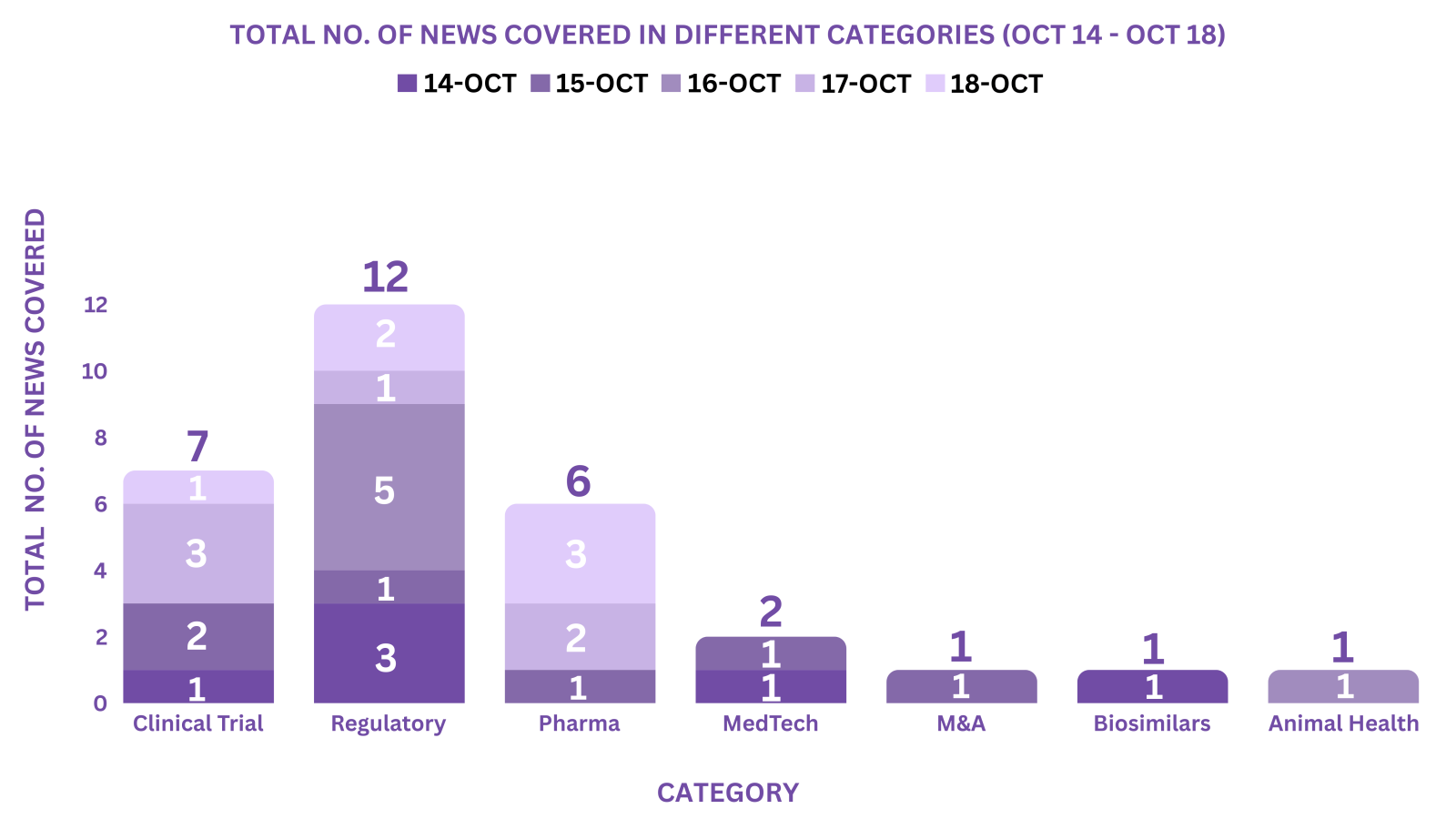
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Biosimilars & Animal Health. Check out our full report below:

Roche Highlights 2-Years Data from RAINBOWFISH Trial of Evrysdi for Treating Children with Spinal Muscular Atrophy (SMA) at WMS 2024
Read More: Roche
Eli Lilly Highlights 1 Year Analysis from the P-III (VIVID-1) Trial of Mirikizumab for Treating Crohn's Disease at UEG Week 2024
Read More: Eli Lilly
Circle Pharma Reports the First Patient Dosing with CID-078 Under P-I Study for Advanced Solid Tumors
Read More: Circle Pharma
Innovent Biologics Reports the P-II Trial Data of Picankibart (IBI112) in Chinese Patients with Ulcerative Colitis
Read More: Innovent Biologics
Marinus Pharmaceuticals Highlights the P-III (RAISE) Study Data of Ganaxolone to Treat Refractory Status Epilepticus (RSE) at NCS 2024
Read More: Marinus Pharmaceuticals
Latigo Biotherapeutics Reports the First Patient Dosing with LTG-305 in its P-I Study for Non-Opioid Treatment of Pain
Read More: Latigo Biotherapeutics
Merck Highlights the P-IIb/III (MK-1654-004) Study Data of Clesrovimab to Prevent RSV Disease in Healthy Preterm and Full-term Infants at IDWeek 2024
Read More: Merck

Pfizer Reports the US FDA’s Approval of Hympavzi to Treat Hemophilia A/B without Inhibitors in Adults and Adolescents
Read More: Pfizer
AstraZeneca and Daiichi Sankyo Report the NMPA’s Conditional Approval of Enhertu to Treat Metastatic NSCLC with HER2 Mutations
Read More: AstraZeneca and Daiichi Sankyo
Bayer Reports the Regulatory Submission of Nubeqa (Darolutamide) to the EMA for Treating Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)
Read More: Bayer
Dizal’s Sunvozertinib Receives the NMPA’s Breakthrough Therapy Designation as a 1L Treatment of NSCLC with EGFR Mutations
Read More: Dizal
Novocure’s Optune Lua Receives the US FDA’s Nod for Treating Metastatic Non-Small Cell Lung Cancer
Read More: Novocure
UroGen Reports the US FDA’s NDA Acceptance of Mitomycin (UGN-102) for Treating Non-Muscle Invasive Bladder Cancer
Read More: UroGen
GSK Reports the US FDA’s NDA Acceptance of Gepotidacin with Priority Review to Treat Uncomplicated Urinary Tract Infections
Read More: GSK
Lantern Pharma’s LP-184 Gains the US FDA’s Fast-Track Designation for Treating Glioblastoma
Read More: Lantern Pharma
Cullinan Therapeutics Reports the US FDA’s IND Approval of CLN-978 to Treat Systemic Lupus Erythematosus
Read More: Cullinan Therapeutics
Alnylam Reports the Submission of Regulatory Application of Amvuttra (Vutrisiran) to the EMA Treating ATTR Amyloidosis with Cardiomyopathy
Read More: Alnylam Pharmaceuticals
AbbVie Reports the US FDA’s Approval of Vyalev (Foscarbidopa and Foslevodopa) for Treating Advanced Parkinson's Disease
Read More: AbbVie
Novartis’ Kisqali (Ribociclib) Gains the CHMP’s Positive Opinion as an Adjuvant Treatment of HR+/HER2- Early Breast Cancer (EBC)
Read More: Novartis

Exelixis and Merck Join Forces to Develop Zanzalintinib for Head and Neck Cancer and Renal Cell Carcinoma
Read More: Exelixis and Merck
Viatris Join Forces with Lexicon Pharmaceuticals to Commercialize Sotagliflozin Across all Markets Outside of the US and EU
Read More: Viatris and Lexicon Pharmaceuticals
Sanofi Collaborates with Orano Med for the Development of Next-Generation Radioligand Therapies
Read More: Sanofi and Orano Med
Factor Bioscience Collaborates with Eterna Therapeutics to Develop Cell Therapy Focusing on Oncology, Autoimmune and Rare Diseases
Read More: Factor Bioscience and Eterna Therapeutics
Poseida Therapeutics Nominates a New CAR-T Development Candidate Under Partnership with Roche
Read More: Poseida Therapeutics and Roche
LaNova Medicines Reports the Commencement of P-I Study Evaluating LM-299 for Solid Tumors and Completes Series C1 Financing Round
Read More: LaNova Medicines

Abbott Reports the Study Advancements in Pulsed Field Ablation, Gets the US FDA’s Nod for Technology Supporting Cardiac Mapping
Read More: Abbott
Bausch + Lomb Reports the US FDA’s Approval of enVista Envy Full Range of Vision Intraocular Lens
Read More: Bausch + Lomb

Lundbeck to Acquire Longboard Pharmaceuticals, Strengthening its Neuroscience Portfolio
Read More: Lundbeck and Longboard Pharmaceuticals

Dong-A ST Reports the US FDA’s Approval of Imuldosa (Biosimilar, Stelara)
Read More: Dong-A ST

Merck Animal Health Reports the EMA’s Approval of an Expanded Indication for SC Administration of Bovilis Rotavec Corona
Read More: Merck Animal Health
Related Post: PharmaShots Weekly Snapshots (October 07 – October 11, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














