Role of Wearables in Cardiovascular Domain
The heart is the muscular organ that pumps blood throughout the body. A group of diseases that affect the heart and blood vessels of the body comes under cardiovascular disease (CVD). Heart disease is globally the major cause of death (~17.3M). Irregular heart functions can lead to heart arrhythmia, heart attack, myocardial infarction, and other complications. Nowadays wearable devices are demanded over other traditional devices for an uninterrupted and to avoid the complexity and inconvenience of ECG and wrist pulse monitoring1
ECG (Electrocardiogram) is the test to record the heart impulse in which electrodes are placed on the chest, arms, and legs and connected to an ECG machine by lead wires then measured, and printed out the electrical activity of the heart.
The market of smart wearable devices is very vast and are available in the form of ECG patches, chest strap, smartwatches or bands, clothing, shoe-embedded sensors and rings which is based on accelerometer, barometer, GPS, PPG, or ECG. A practical ABCD clinician’s guide can facilitate the integration of these devices into the clinical workplace6
A wearable device, CNT-PDMS-based flexible sensors was developed to simultaneously measure wrist pulse pressure through a strain sensor and ECG signals via flexible electrodes. Wireless communication (e.g. Bluetooth) would be used to transmit the data captured by the device to a mobile phone for wireless monitoring to the server and analysed to provide early warnings of cardiac diseases1
There are multiple applications for wearables in the field of CVD:
- Heart failure management
- Acute coronary syndrome diagnosis
- Cardiac tetrahabilitation
- Wearable devices in cardiovascular clinical studies
- Hypertension screening and management
- Measurement of Atrial fibrillation and other arrhythmias
The below graph shows no. of devices that measures various biological measurements incl. HR, ECG, PA, BP, SPO2 & Sleep. For ex: 13 watches measure HR & PA, 3 measure ECG measure SPO2, 7 measures sleep, and 0 watches measures BP
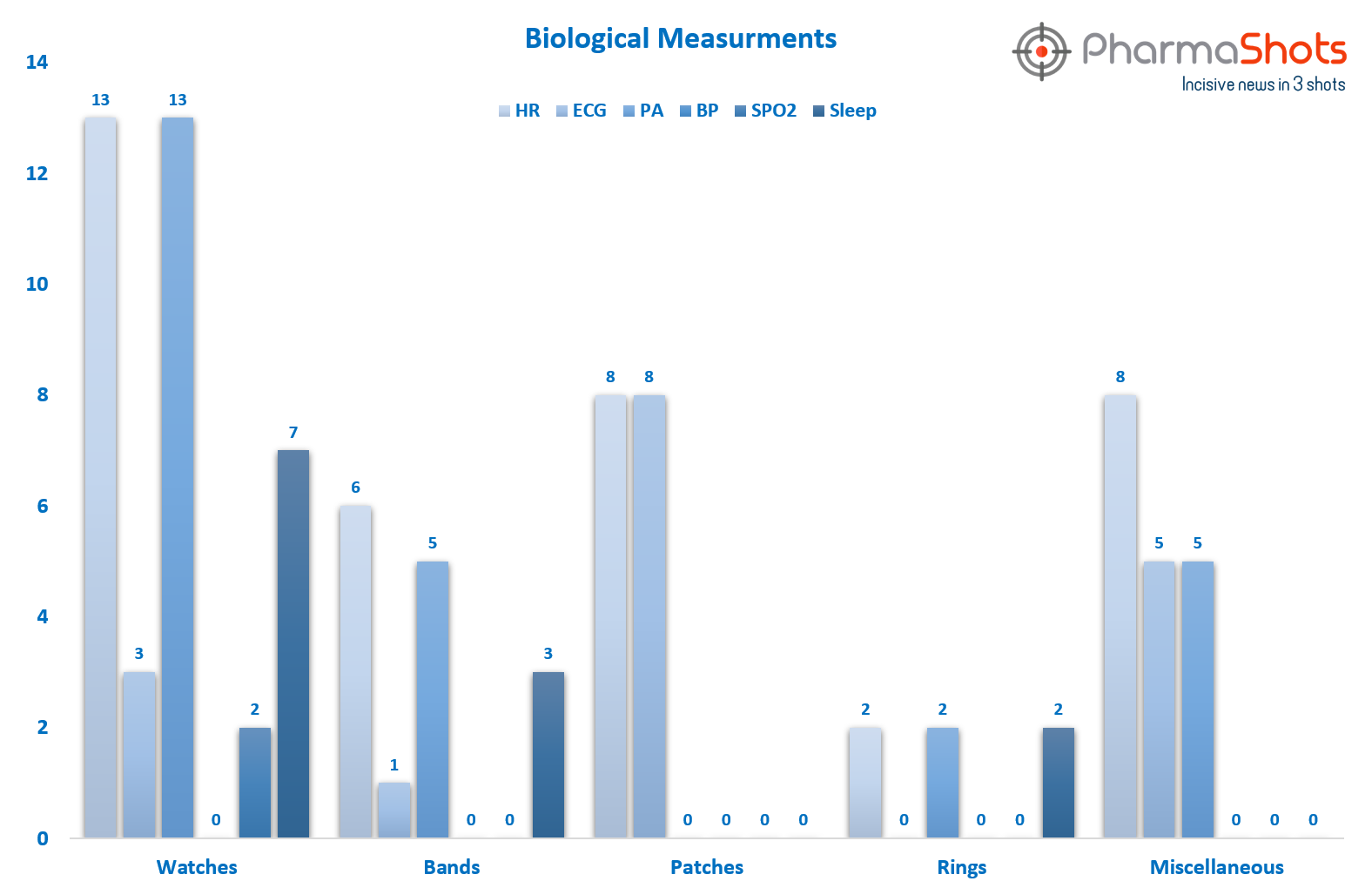
Figure1: Few of the commonly tracked biologicals activities by these wearables may include: HR: Heart Rate, PA: Physical Activity; SpO2: Oxygen saturation; ECG: Electrocardiogram; BP: Blood Pressure

Figure2: Provides information on the type devices, what types of sensors have been built in and what do they measure or assess and what are its clinical application and uses. Image Source6
There are approximately number of watches (21), Patches (8), Bands (4), Rings (2) and some miscellaneous products (10) and respectively the trials (80, 24, 6, 0, 17) in which these wearables are being utilized
The key companies with maximum number of trials involving wearable devices are- Motiv (motiv ring) & Oura (oura ring) for rings, Microsoft for bands, iRhythm (Zio Patch) for patches and Fitbit (Flex, One, Charge) for watches.

Figure 3: Shows the Categories, their numbers and number of trials being running in the same
By using smartphone and AI, various companies are engaged is developing wearable health monitoring devices and smartphone apps for 24/7 health tracking, and to reduce the hospital visits. Key players in the wearable devices includes6

Table 1: List of Devices Along with their Categories and Companies*
A digital health company Eko has recently launched its newly-redesigned Eko App in 2020 installed on a mobile device, healthcare providers who use the company’s digital stethoscopes will be able to analyse readings with Eko’s AI algorithms and transforms the traditional stethoscope into an intelligent disease detection tool to help clinicians to easily find out the CVD during the physical exam2
Sensydia is developing a new cardiac device CPS (Cardiac Performance System) that recently received BDD from the US FDA in Jan 2022. It measures the critical heart performance and reports to physicians to evaluate heart failure conditions3
Alivecor has developed Kardia Mobile 6L, the FDA-cleared six-lead personal ECG device that monitors the heart at home and detects the 3 most common heart arrhythmias like atrial fibrillation, bradycardia, and tachycardia
Matrix’s Power watch 2 is a thermoelectric power smartwatch that works when it gets the body heat of the wearer and it consists of a pedometer, heart rate monitor, GPS, sleep tracker, and a corresponding app4
In Feb 2021, RhythMedix launched the newest-generation wearable cardiac monitor, the RhythmStar that itself can collect ECG recordings and send them wirelessly to physicians without the need for an intermediate device such as a phone5
Recently, Design Partners launched Viscero, a wearable ECG monitoring vest to measure heartbeats. This technology could replace the Holter monitor. It can be worn under clothes and no discomfort causes to the wearer, while results are captured via a doctor-patient dashboard, to enable further simplification7
Zoll Medical has launched Zoll µCor HFAMS non-invasive patch-based wireless system for heart failure and arrhythmia that measures the pulmonary fluid levels by using radiofrequency technology. The data were analysed by the company’s algorithms. It gives alert messages about the patient’s condition to help the physician in timely identification and diagnosis of clinical conditions8
Conclusion: As technology is experiencing great advancement, the therapeutic and diagnostic options for the health sector have also evolved. Today a patient and caregiver (incl. HCP) has many options by which they can maintain & monitor health vitals. Wearables are a great asset in the field of healthcare that has helped in many ways in the diagnosis of cardiovascular diseases. Although, there are many challenges that restrict the extensive use of wearable technologies in clinical practice
*This is not an exhaustive list
Reference:
- http://amnl.mie.utoronto.ca/data/J193.pdf
- https://www.ekohealth.com/newsroom/eko-launches-screening-solution-to-help-catch-heart-disease-early
- https://www.businesswire.com/news/home/20220119005413/en/Sensydia-Receives-FDA-Breakthrough-Device-Designation-for-CPSTM-Non-Invasive-Cardiac-Monitoring-Device
- https://www.powerwatch.com/
- https://www.medicaldevice-network.com/comment/rhythmedix-cardiac-monitor/
- https://www.nature.com/articles/s41569-021-00522-7/figures/1
- https://www.healthtechdigital.com/wearable-ecg-vest-from-design-partners-takes-the-pain-out-of-heart-monitoring/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7343723/#:~:text=Most%20wearables%20are%20marketed%20to,support%20exercise%20training%20and%20rehabilitation.
Related Post: FTC Anti-Trust Policy: Future of Pharmaceutical Industry



