PharmaShots Weekly Snapshots (Sep 22, 2025 – Sep 26, 2025)
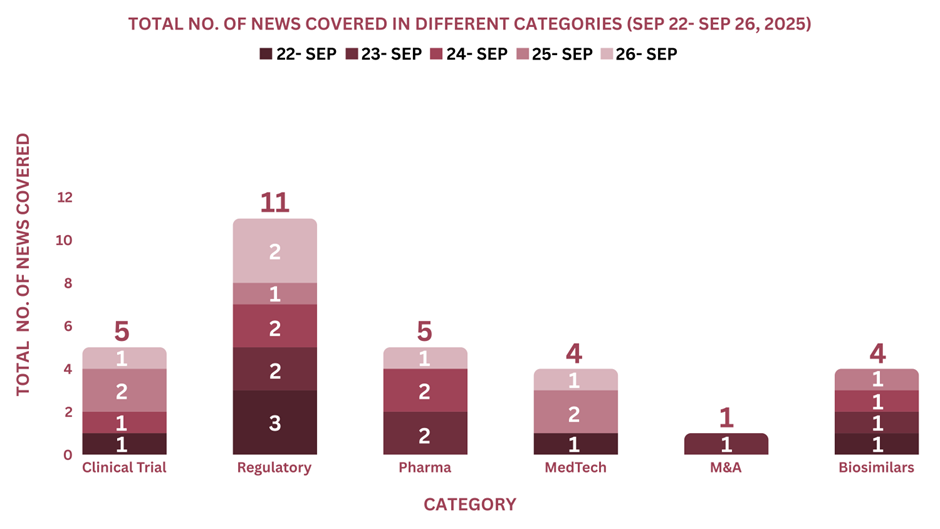
This week, PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A and Biosimilars. Check out our full report below:

Roche Reports P-III (evERA Breast Cancer) Trial Data on Giredestrant + Everolimus for ER-Positive Breast Cancer
Read More: Roche
BMS Reports the P-III (EXCALIBER-RRMM) Trial Data of Iberdomide Regimen to Treat R/R Multiple Myeloma
Read More: BMS
Hansa Biopharma Reports Topline P-III (ConfIdeS) Trial Data of Imlifidase in Kidney Transplantation
Read More: Hansa Biopharma
Acadia Pharmaceuticals Reports Topline P-III (COMPASS PWS) Trial Data of Carbetocin for Hyperphagia in Prader-Willi Syndrome
Read More: Acadia Pharmaceuticals
Intellia Reports Long-Term P-I Trial Data of Nexiguran Ziclumeran for Hereditary ATTR Amyloidosis with Polyneuropathy (ATTRv-PN)
Read More: Intellia Therapeutics

Johnson & Johnson Reports the CHMP’s Positive Opinion of Nipocalimab for Generalized Myasthenia Gravis (gMG)
Read More: J&J
The CHMP Adopts Positive Opinion on Merck’s Enflonsia for RSV Prevention in Infants
Read More: Merck
Merck’s Keytruda Qlex Receives the US FDA’s Approval for Subcutaneous Use Across 38 Solid Tumor Indications for Keytruda
Read More: Merck
Bayer Reports the CHMP’s Positive Opinion of Elinzanetant to Treat Vasomotor Symptoms
Read More: Bayer
Ionis’ Tryngolza (Olezarsen) Receives the EC’s Approval for Familial Chylomicronemia Syndrome
Read More: Ionis
AstraZeneca and Amgen Report the CHMP’s Positive Opinion of Tezspire (Tezepelumab) for CRSwNP
Read More: AstraZeneca and Amgen
Daiichi Sankyo and AstraZeneca Receive the US FDA’s Priority Review for Enhertu + Perjeta to Treat HER2+ Breast Cancer
Read More: Daiichi Sankyo & AstraZeneca
Artios’ Alnodesertib Obtains the US FDA’s Fast Track Designation for ATM-negative Metastatic Colorectal Cancer
Read More: Artios
Eli Lilly Reports Kisunla (Donanemab) Approval in the EU to Treat Early Symptomatic Alzheimer’s Disease
Read More: Eli Lilly
Crinetics Pharmaceuticals Reports the US FDA’s Approval of Palsonify (Paltusotine) for Acromegaly
Read More: Crinetics Pharmaceuticals
Eli Lilly’s Inluriyo (Imlunestrant) Receives the US FDA’s Approval for ESR1-mutated Breast Cancer
Read More: Eli Lilly

Starpharma Inks a ~$569.5M Deal with Genentech to Develop Cancer Therapies
Read More: Starpharma and Genentech
Angelini Pharma Enters an Exclusive Option Agreement with Sovargen to Develop and Commercialize SVG105 for Brain Disorders
Read More: Angelini Pharma and Sovargen
Variational AI Collaborates with Merck to Develop Small Molecule Therapeutics
Read More: Variational AI and Merck
Solid Biosciences & Kinea Bio Partner to Advance KNA-155 Using AAV-SLB101 for Dysferlin-Related Limb-Girdle Muscular Dystrophy
Read More: Solid Biosciences & Kinea Bio
Evaxion Licenses EVX-B3 Vaccine Candidate to Merck
Read More: Evaxion and Merck

Medtronic’s Altaviva Device Receives the US FDA’s Approval to Treat Urge Urinary Incontinence
Read More: Medtronic
Smart Meter Launches iAmbientHealth for Remote Patient Monitoring
Read More: Smart Meter
ClearNote Health Receives UKCA Marking for its Avantect Multi-Cancer & Ovarian Cancer Detection Tests
Read More: ClearNote Health
HemoSonics Secures the US FDA’s 510(k) Clearance for Quantra Hemostasis System in Obstetric Procedures
Read More: HemoSonics

Pfizer Expands into Obesity Treatments with ~$7.3B Metsera Acquisition
Read More: Pfizer & Metsera

Henlius and Organon Receive the EC’s Approval for Bildyos & Bilprevda (Biosimilars, Prolia & Xgeva)
Read More: Henlius & Organon
Alvotech Receives the CHMP’s Positive Opinion for AVT03 (Biosimilar, Prolia & Xgeva)
Read More: Alvotech
Alvotech’s Gobivaz (Biosimilar, Simponi) Receives the CHMP’s Positive Opinion to Treat Several Chronic Inflammatory Diseases
Read More: Alvotech
Kashiv BioSciences and JAMP Pharma Launch Pexegra (Biosimilar, Neulasta) and Filra (Biosimilar, Neupogen) in Canada
Read More: Kashiv BioSciences & JAMP Pharma
Related Post: PharmaShots Weekly Snapshots (Sep 15, 2025 – Sep 19, 2025)

