PharmaShots Weekly Snapshots (Aug 18, 2025 – Aug 22, 2025)
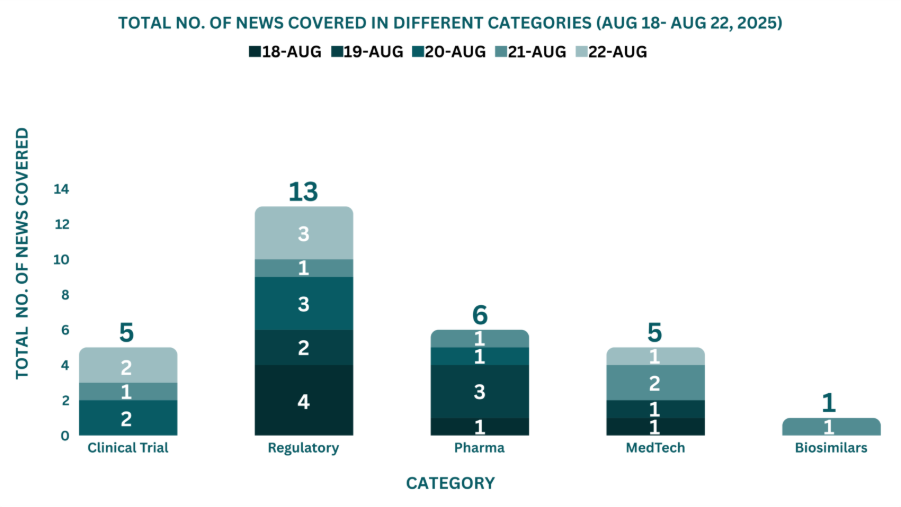
This week, PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, and Biosimilars. Check out our full report below:

HUTCHMED Completes Patient Enrolment in P-III (SANOVO) Trial of Orpathys (savolitinib) and Tagrisso (osimertinib) for EGFRm NSCLC
Read More: HUTCHMED
Akeso Reports First Patient Dosing in P-III (COMPASSION-33) Trial of Cadonilimab for G/GEJ Adenocarcinoma
Read More: Akeso
Arthrosi Therapeutics Reports Full Enrolment in P-III (REDUCE 1) Trial of Pozdeutinurad for Gout
Read More: Arthrosi Therapeutics
AbbVie Reports Topline P-III (UP-AA) Trial Data on Rinvoq (Upadacitinib) to Treat Alopecia Areata
Read More: AbbVie
Tyra Biosciences Doses First Patient in P-II (BEACH301) Trial of Dabogratinib to Treat Achondroplasia
Read More: Tyra Biosciences

Precigen’s Papzimeos Receives the US FDA’s Full Approval for Recurrent Respiratory Papillomatosis
Read More: Precigen
Novo Nordisk Reports the US FDA’s Approval of Wegovy for Metabolic Dysfunction-Associated Steatohepatitis (MASH)
Read More: Novo Nordisk
Valneva Obtains Health Canada’s Approval for Ixchiq to Prevent Chikungunya
Read More: Valneva
SystImmune and BMS’ Izalontamab Brengitecan Secures the US FDA’s Breakthrough Therapy Designation to Treat EGFRm NSCLC
Read More: SystImmune and BMS
SpringWorks Therapeutics (Merck KGaA) Reports the EC’s Approval of Ogsiveo (Nirogacestat) for Desmoid Tumors
Read More: SpringWorks Therapeutics
Tonix Pharmaceuticals’ Tonmya Receives the US FDA’s Approval for Fibromyalgia
Read More: Tonix Pharmaceuticals
Madrigal Pharmaceuticals’ Rezdiffra (Resmetirom) Receives the EC’s Conditional Approval for MASH with Liver Fibrosis
Read More: Madrigal Pharmaceuticals
Health Canada Grants Conditional Approval to Iovance’s Amtagvi (Lifileucel) for Advanced Melanoma
Read More: Iovance
Novo Nordisk Reports the Health Canada’s Approval of Ozempic to Reduce Risk of Kidney Disease Progression & Cardiovascular Death in T2D & CKD Adults
Read More: Novo Nordisk
Rhythm Pharmaceuticals Reports the US FDA’s sNDA Submission of Setmelanotide for Acquired Hypothalamic Obesity
Read More: Rhythm Pharmaceuticals
Ionis Reports the US FDA’s Approval of Dawnzera (Donidalorsen) as a Prophylactic Treatment of Hereditary Angioedema (HAE)
Read More: Ionis
Bayer Reports Health Canada’s Approval of Nubeqa for Treating Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)
Read More: Bayer
Health Canada Approves Roche’s Columvi (Glofitamab) Combination to Treat R/R Diffuse Large B-Cell Lymphoma (DLBCL)
Read More: Roche

Eli Lilly Collaborates with Superluminal Medicines to Develop Small Molecule for Cardiometabolic Diseases and Obesity
Read More: Eli Lilly and Superluminal Medicines
Skyhawk Therapeutics Enters a ~$2B Deal with Merck KGaA to Identify RNA-Targeting Small Molecules for Neurological Disorders
Read More: Skyhawk Therapeutics and Merck KGaA
Boehringer Ingelheim Joins Forces with Palatin to Develop Novel Therapies for Retinal Diseases
Read More: BI and Palatin
RemeGen Enters a Licensing Agreement with Santen Pharmaceutical for RC28-E to Treat Eye Diseases
Read More: RemeGen and Santen Pharmaceutical
VantAI Collaborates with Halda Therapeutics to Discover RIPTAC Medicines
Read More: VantAI and Halda Therapeutics
Jazz Pharmaceuticals Enters a ~$1.03B Licensing Deal with Saniona for SAN2355
Read More: Jazz Pharmaceuticals and Saniona

Galvanize Therapeutics Enrolls First Patient in PROPEL Registry Trial of Aliya Pulsed Electric Field Ablation for Soft Tissue Lesions
Read More: Galvanize Therapeutics
ONWARD Medical Receives FDA IDE Approval for ARC-IM System to Treat Blood pressure Instability in Spinal Cord Injury
Read More: ONWARD Medical
neurocare Receives the US FDA’s Clearance for Apollo TMS Therapy Devices to Treat Obsessive Compulsive Disorder
Read More: neurocare
Agilent Technologies Reports the US FDA’s Approval of MMR IHC Panel pharmDx (Dako Omnis) as a Companion Diagnostic Test for Colorectal Cancer
Read More: Agilent Technologies
Artrya Receives the US FDA’s 510(k) Clearance for Salix Coronary Plaque Module to Detect Coronary Artery Plaque
Read More: Artrya

Alvotech and Advanz Pharma Receive the EC’s Approval for Mynzepli (Biosimilar, Eylea)
Read More: Alvotech and Advanz Pharma
Related Post: PharmaShots Weekly Snapshots (Aug 11, 2025 – Aug 14, 2025)

