EMA Marketing Authorization of New Drugs in July 2025
Shots:
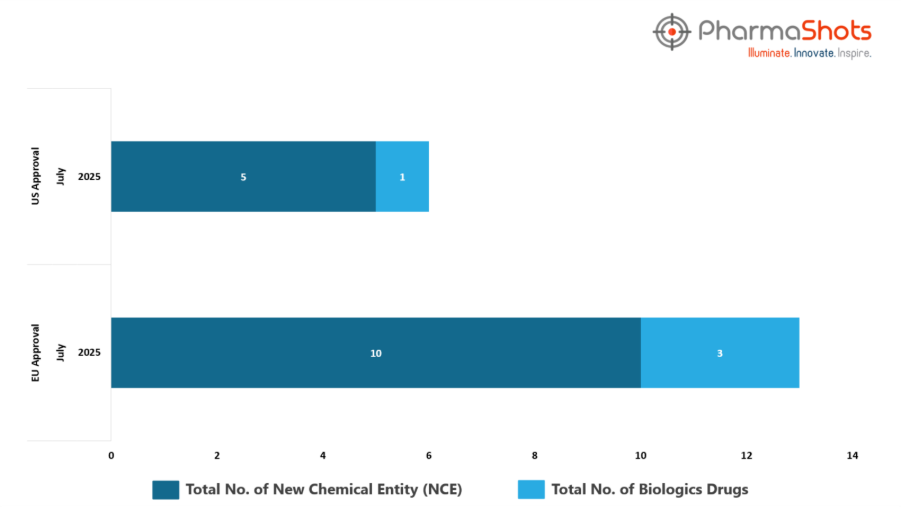
- The EMA’s CHMP has granted positive opinions and approvals to 3 Biologics and 10 new chemical entities in July 2025, leading to treatments for patients and advances in the healthcare industry
- The major highlighted drug was Roche’s Itovebi Secures the EC’s Approval for PIK3CA-mutated Breast Cancer
- PharmaShots has compiled a list of 13 drugs that have been granted positive opinions and approvals by the EC, respectively
Company: Vertex Pharmaceuticals
Product: Alyftrek
Active Ingredient: Deutivacaftor/Tezacaftor/Vanzacaftor
Disease: Cystic Fibrosis
Date: Jul 01, 2025
Shots:
- The EC has approved Alyftrek (deutivacaftor/tezacaftor/vanzacaftor) to treat pts (≥6yrs.) with cystic fibrosis (CF), having ≥1 non-class I mutation in the CFTR gene; regulatory review is ongoing in Canada, Switzerland, Australia, & New Zealand
- In 2 H2H trials, Alyftrek + ivacaftor matched Kaftrio (ivacaftor/tezacaftor/ elexacaftor) on ppFEV1 & showed superiority in reducing sweat chloride in CF pts
- Vanzacaftor & tezacaftor increase CFTR protein at the cell surface, while deutivacaftor enhances its function by boosting channel open probability to improve salt & water flow
Company: SpringWorks Therapeutics
Product: Ezmekly
Active Ingredient: Mirdametinib
Disease: Neurofibromatosis Type 1 Associated Symptomatic Plexiform Neurofibromas
Date: Jul 18, 2025
Shots:
- The EC has granted conditional approval to Ezmekly (mirdametinib) for pts (≥2yrs.) with unresectable neurofibromatosis type 1 associated symptomatic plexiform neurofibromas (NF1-PN) based on P-IIb (ReNeu) trial
- The P-IIb (ReNeu) study assessed mirdametinib (2mg/m^2, BID) in 2 Arms (N=114: 56 pediatric & 58 adults) & met its 1EP of cORR (52% & 41%) with durable tumour volume reduction of -42% (Range: -91 to 48%) & -41% (Range: -90 to 13%)
- 90% children & 88% adults had response of at least 12mos., while 48% & 50% had responses for ≥24 mos. Both groups showed early, sustained pain relief & improved quality of life; data was shared at ASCO 2024
Company: Autolus Therapeutics
Product: Aucatzyl
Active Ingredient: Obecabtagene Autoleucel
Disease: R/R B-Cell Acute Lymphoblastic Leukemia
Date: Jul 21, 2025
Shots:
- The EC has approved Aucatzyl (Obecabtagene Autoleucel) for treating pts (≥26yrs.) with r/r B-ALL in all 30 EEA states
- The EC approval was based on the FELIX study (n=100+), which showed that pts in cohort IIA (n=94) receiving ≥1 infusion of obe-cel had a 76.6% CR/CRi rate, with a mDoR of 21.2mos. & mEFS of 11.9mos.; 6 & 12mos. EFS rates were 65.4% & 49.5%, respectively. Data was published in the NEJM.
- Obe-cel is an autologous CD19 CAR T cell therapy with a proprietary CAR, approved by the FDA and authorized by the UK MHRA
Company: Roche
Product: Itovebi
Active Ingredient: Inavolisib
Disease: PIK3CA-mutated, ER+/HER2- Locally Advanced or Metastatic Breast Cancer
Date: Jul 23, 2025
Shots:
- The EC has approved Itovebi + Ibrance & fulvestrant as a 1L therapy for adults with PIK3CA-mutated, ER+/HER2- locally advanced or metastatic breast cancer recurring on or within 12mos. of adj. endocrine therapy, based on the P-III (INAVO120) trial
- Trial (n=325) assessed the regimen vs PBO + Ibrance & fulvestrant, which showed improved PFS (1EP) by 57% (mPFS: 15 vs 7.3mos.) & a 33% reduction in death risk, with consistent PFS benefit across subgroups; regimen delayed also CT by ~2yrs. PFS & OS data was published in The NEJM, with OS data presented at ASCO 2025
- Additionally, Roche is evaluating Itovebi in 3 more P-III trials (INAVO121, INAVO122 & INAVO123) for the same indication in various combinations
Company: GSK
Product: Blenrep
Active Ingredient: Belantamab Mafodotin
Disease: R/R Multiple Myeloma
Date: Jul 23, 2025
Shots:
- The EC has approved Blenrep + BorDex & PomDex in MM pts with ≥1 prior therapy. Review ongoing in the US (PDUFA: Oct 23, 2025) & China (priority review for DREAMM-7)
- Approval was based on P-III (DREAMM-7 & DREAMM-8) trials assessing Blenrep + BorDex vs Darzalex + BorDex in 494 pts & Blenrep + PomDex (BPd) vs Velcade + PomDex in 302 pts, respectively
- DREAMM-7 showed improved PFS (1EP; mPFS: 36.6 vs 13.4mos.), depicting a 42% reduction in death risk at 39.4 mFU & 3yr. OS rate (2EP) of 74% vs 60% (mOS: unreached for both arm), while DREAMM-8 showed unreached mPFS vs 12.7mos. at mFU of 21.8mos.
Company: Servier
Product: Voranigo
Active Ingredient: Vorasidenib
Disease: Grade 2 IDH-Mutant Glioma
Date: Jul 24, 2025
Shots:
- The CHMP has recommended to Voranigo for treating patients (age: ≥12yrs.; wt≥ 40kg) with Gr2 astrocytoma or oligodendroglioma with a susceptible IDH1/2 mutation post-surgery but do not currently require radiotherapy or CT; anticipated EC decision in all 30 EEA states
- The CHMP opinion is based on the global P-III (INDIGO) trial (N=331), which included VORANIGO (n=168) or PBO (n=163). Among them, oligodendroglioma (n=172; 88 Voranigo; 84 PBO) and astrocytoma (n=159; 80 Voranigo and 79 PBO) were presented at ASCO’23 and published in the NEJM
- Voranigo (40mg Tablets) is an isocitrate dehydrogenase-1 (IDH1) and isocitrate dehydrogenase-2 (IDH2) inhibitor
Company: KalVista Pharmaceuticals
Product: Ekterly
Active Ingredient: Sebetralstat
Disease: Hereditary Angioedema
Date: Jul 24, 2025
Shots:
- The CHMP has recommended sebetralstat to treat acute attacks of hereditary angioedema (HAE) pts (≥12yrs.); EC’s decision expected by early Oct 2025. Application is under review in Japan & other regions
- Approval was based on P-III (KONFIDENT) study assessing sebetralstat (300mg & 600mg) vs PBO in 136 HAE pts (≥12yrs.) across 20 countries
- KONFIDENT data was published in The NEJM, which showed faster symptom relief, reduced attack severity & resolution
Company: Biogen
Product: Zurzuvae
Active Ingredient: Zuranolone
Disease: Severe Postpartum Depression
Date: Jul 24, 2025
Shots:
- The CHMP has recommended Zurzuvae (zuranolone) for the treatment of postpartum depression in adults; EC’s decision is expected in Q3’25
- Approval was based on P-III (SKYLARK) trial assessing Zurzuvae (50mg) vs PBO in pts with severe postpartum depression
- Trial met its 1EP with a significant mean reduction in HAMD-17 total score at Day 15, & all key 2EP, which showed a rapid reduction in depressive symptoms by Day 3, sustained through Day 45
Company: Gilead
Product: Yeytuo
Active Ingredient: Lenacapavir
Disease: Pre-Exposure Prophylaxis to Prevent HIV
Date: Jul 24, 2025
Shots:
- The CHMP has recommended approval of the MAA & EU-M4all application of lenacapavir as PrEP for individuals at risk of HIV across all 30 EEA states. It will be marketed in the EU as Yeytuo, if approved by the EC by late 2025
- Opinion was based on P-III (PURPOSE 1 & PURPOSE 2) trials assessing lenacapavir (SC; Q6M) vs Truvada (F/TDF; PO, QD), where PURPOSE 1 focussed on cisgender women, while PURPOSE 2 assessed gender-diverse individuals incl. geographically diverse range of cisgender men
- PURPOSE 1 showed 0 infections & 100% risk reduction in 2134 women, while PURPOSE 2 showed 99.9% non-infection rate in 2179 pts (2 acquired HIV), demonstrating superiority in contrast to bHIV in both trials; data was published in The NEJM
Company: Deciphera Pharmaceuticals
Product: Romvimza
Active Ingredient: Vimseltinib
Disease: Symptomatic Tenosynovial Giant Cell Tumor
Date: Jul 24, 2025
Shots:
- The CHMP has recommended Romvimza (vimseltinib) to treat symptomatic TGCT with physical function deterioration in adults for which surgery may lead to functional impairment or severe morbidity; EC’s decision is expected in Q3’25
- Opinion was based on P-I/II trial as well as P-III (MOTION) trial, which evaluated Romvimza vs PBO in surgery-ineligible pts without prior anti-CSF1/CSF1R therapy (prior imatinib/nilotinib allowed)
- The P-III trial met its 1EP of improved ORR (40% vs 0%) in ITT pts, plus showed improvements across active range of motion, patient-reported physical functioning, & pain observed at 25wks., with favourable safety as depicted by P-I/II data
Company: Ionis
Product: Tryngolza
Active Ingredient: Olezarsen
Disease: Familial Chylomicronemia Syndrome
Date: Jul 24, 2025
Shots:
- The CHMP has recommended Tryngolza (olezarsen) as an adjunct to diet in adult patients for the treatment of genetically confirmed FCS; EC’s decision is expected in Q4’25
- Opinion was based on P-III (Balance) trial assessing Olezarsen (Q4W) vs PBO, which showed reduced triglyceride levels at 6mos., sustained through 12mos., with decrease in acute pancreatitis events over 12mos.; data was published in The NEJM
- Tryngolza is also being evaluated in the P-III (CORE & CORE2) trials for sHTG, with data expected in Q3’25
Company: IntraBio
Product: Aqneursa
Active Ingredient: Levacetylleucine
Disease: Niemann-Pick Disease Type C
Date: Jul 24, 2025
Shots:
- The CHMP has recommended Aqneursa (levacetylleucine) for treating adults & pediatric pts with NPC; additional global submissions are planned for 2025 & beyond
- Approval was backed by P-III (IB1001-301) study assessing Aqneursa vs PBO in pts (≥4yrs.; n=60) with NPC, which showed improved neurological symptoms & function across all 1 & 2EPs within 12wks., with LTE phase showing disease-modifying & neuroprotective effects. IB1001-301 trial data was published in The NEJM
- Also, Aqneursa is being evaluated in a P-III trial for Ataxia-Telangiectasia, which completed recruitment in under 2mos., over-enrolled by 167%, & is expected to report data in Q1’26
Company: Purpose Pharma
Product: Attrogy
Active Ingredient: Diflunisal
Disease: Hereditary Transthyretin-mediated Amyloidosis
Date: Jul 31, 2025
Shots:
- The EC has approved Attrogy (diflunisal) to treat hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis) in adults with stage 1 or 2 polyneuropathy in all EEA states
- EC approval was based on a P-III trial showing that diflunisal significantly slowed disease progression in patients with TTR amyloidosis. At 24 mos. baseline, 1EP met diflunisal led to a mean NIS+7 increase of 8.2 points vs. 26.3 with PBO. 2EPs, including NIS, NIS-LL, Kumamoto score, and SF-36 physical score, also showed significant benefits vs PBO
- Diflunisal (250 mg BID), a difluorophenyl salicylic acid derivative, stabilizes the TTR tetramer
Related Post: Insights+: EMA Marketing Authorization of New Drugs in June 2025



