PharmaShots Weekly Snapshots (Jun 30, 2025 – Jul 04, 2025)
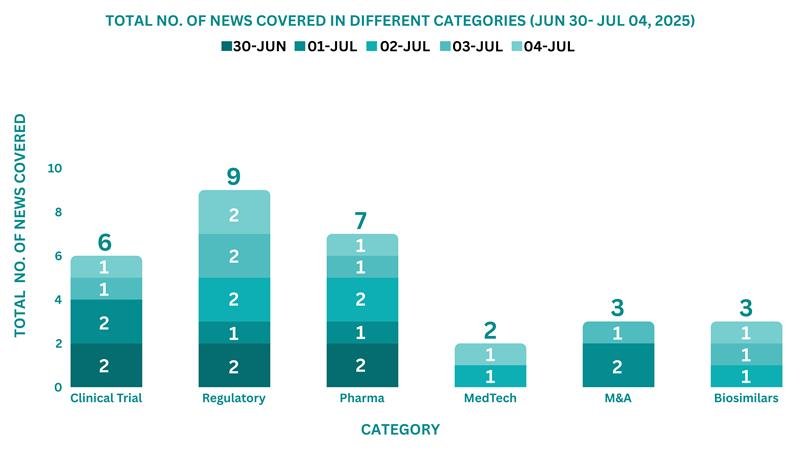
This week, PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, and Biosimilar. Check out our full report below:

Neurocrine Reports Data From KINECT-HD Study Evaluating INGREZZA in Huntington’s Disease (HD)
Read More: Neurocrine
UCB Reports the P-III Data of Fenfluramine in CDKL5 Deficiency Disorder (CDD) Patients
Read More: UCB
Neurocrine Biosciences Initiates P-I Study of NBIP-01435 to Treat CAH
Read More: Neurocrine Biosciences
Amgen Reports Data from the P-III (FORTITUDE-101) Study Evaluating Bemarituzumab in FGFR2b+ 1L Gastric Cancer
Read More: Amgen
Ascletis Pharma Reports First Patient Dosing with ASC30 in P-IIa Trial for Obese or Overweight Patients
Read More: Ascletis Pharma
Vertex Presents Long-Term CLIMB Trial Program Data of Casgevy (Exagamglogene Autotemcel) to Treat Hemoglobinopathies at EHA 2025
Read More: Vertex Pharmaceutical

Sobi Reports the US FDA Approval of Gamifant for Macrophage Activation Syndrome in Still’s Disease
Read More: Sobi
Omeros Submits MAA to the EMA for Narsoplimab (OMS721) to Treat TA-TMA
Read More: Omeros
Takeda Reports US FDA Approval of GAMMAGARD LIQUID ERC for Primary Immunodeficiency
Read More: Takeda
Jazz Pharmaceuticals’ Ziihera (Zanidatamab) Secures the EC’s Conditional Approval to Treat HER2+ Biliary Tract Cancer (BTC)
Read More: Jazz Pharmaceuticals
Vertex Pharmaceuticals’ Alyftrek Receives the EC’s Approval to Treat Cystic Fibrosis
Read More: Vertex Pharmaceutical
Regeneron’s Lynozyfic (Linvoseltamab-gcpt) Receives the US FDA’s Accelerated Approval for R/R Multiple Myeloma
Read More: Regeneron
Dizal Receives the US FDA’s Accelerated Approval for Zegfrovy (Sunvozertinib) to Treat EGFRm NSCLC
Read More: Dizal
Johnson & Johnson Seeks the EC’s Approval for Akeega to Treat Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)
Read More: J&J
AstraZeneca Reports the EC’s Approval for Perioperative Imfinzi to Treat Muscle-Invasive Bladder Cancer (MIBC)
Read More: AstraZeneca

Mabwell Signs Licensing Agreement with Qilu Pharmaceutical for Albipagrastim Alfa Injection Technology
Read More: Mabwell and Qilu Pharmaceutical
XtalPi and Pfizer Expand Partnership to Advance AI-Driven Drug Discovery and Materials Simulation
Read More: XtalPi and Pfizer
HanchorBio Out-Licenses HCB101 to Shanghai Henlius Biotech For ~$202M
Read More: HanchorBio and Shanghai Henlius Biotech
BioVersys Partners with Shionogi to Jointly Develop Non-Tuberculous Mycobacteria (NTM) Clinical Candidate
Read More: BioVersys and Shionogi
Unnatural Products Collaborates with argenx to Develop Oral Macrocyclic Peptides Across Multiple Indications
Read More: Unnatural Products and argenx
Neurizon Therapeutics Licenses Monepantel from Elanco Animal Health to Accelerate NUZ-001 Commercialization
Read More: Neurizon Therapeutics and Elanco Animal Health
Brii Biosciences Enters a Licensing Deal with Joincare Pharmaceutical for BRII-693 to Treat Bacterial Infections
Read More: Brii Biosciences and Joincare Pharmaceutical

Fasikl Reports the US FDA’s 510(k) Clearance of Felix NeuroAI Wristband to Treat Essential Tremors
Read More: Fasikl
MatOrtho Receives the European CE Mark Approval for ReCerf Hip Resurfacing Arthroplasty
Read More: MatOrtho

AbbVie to Acquire Capstan Therapeutics for ~$2.1B
Read More: AbbVie and Capstan Therapeutics
Torrent Pharma to Acquire a Controlling Stake in JB Chemicals & Pharmaceuticals from KKR for ~$3B
Read More: Torrent Pharma and JB Chemicals & Pharmaceuticals
CB Biotechnology to Acquire Theratechnologies for ~$254M
Read More: CB Biotechnology and Theratechnologies

Alvotech Partners with Advanz Pharma to Commercialize AVT10 (Biosimilar, Cimzia) in the EU
Read More: Alvotech and Advanz Pharma
mAbxience Reports the EC’s Approval of Denbrayce and Izamby (Biosimilar, Xgeva and Prolia)
Read More: mAbxience
Biocon Biologics Receives the EC’s Approval for Vevzuo and Evfraxy (Biosimilars, Xgeva and Prolia)
Read More: Biocon Biologics
Related Post: PharmaShots Weekly Snapshots (Jun 23, 2025 – Jun 27, 2025)

