PharmaShots Weekly Snapshots (Jun 16, 2025 – Jun 20, 2025)
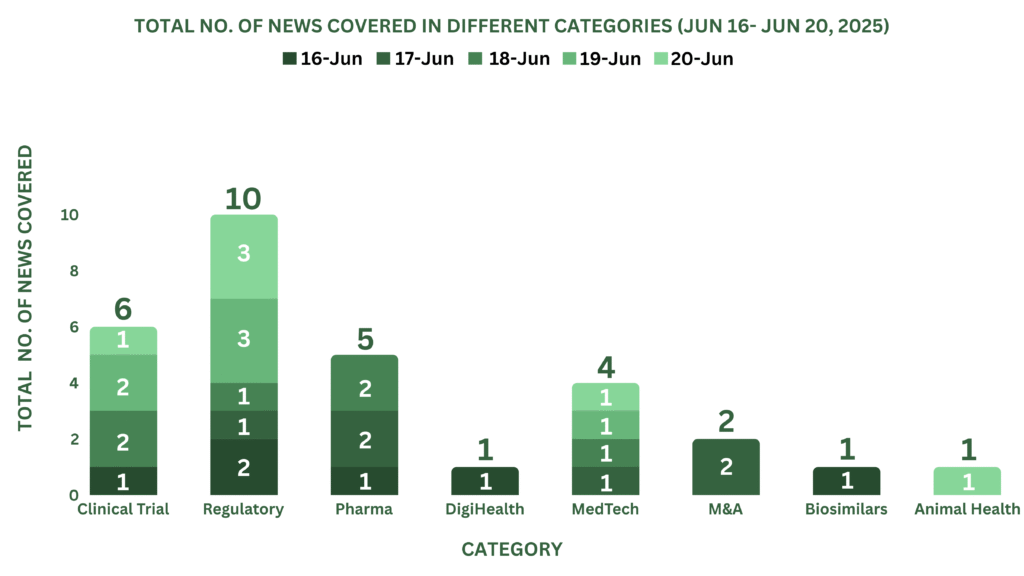
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Biosimilar, Animal Health & DigiHealth. Check out our full report below:

Johnson & Johnson Reports P-II (RedirecTT-1) Trial Data on Talvey + Tecvayli for R/R Multiple Myeloma
Read More: J&J
Camurus Reports Topline P-IIb (POSITANO) Trial Data on CAM2029 for Symptomatic Polycystic Liver Disease
Read More: Camurus
Galderma to Present OLYMPIA Extension Study Data on Nemluvio for Prurigo Nodularis at ICD 2025
Read More: Galderma
AbbVie Reports Topline P-III (TEMPLE) Trial Data on Qulipta (Atogepant) for Migraine Prevention
Read More: AbbVie
Merck and Daiichi Sankyo Report First Patient Dosing with Ifinatamab DXd in P-III (IDeate-Prostate01) Trial for mCRPC
Read More: Merck and Daiichi Sankyo
Dizal Reports Enrollment Completion in P-III (WU-KONG28) Trial of Sunvozertinib for EGFRm NSCLC
Read More: Dizal

Merck Receives the US FDA’s Approval for Perioperative Keytruda for Locally Advanced Head and Neck Squamous Cell Carcinoma (LA-HNSCC)
Read More: Merck
Averoa Receives the EC’s Approval for Xoanacyl to Treat Chronic Kidney Disease
Read More: Averoa
CSL Receives the US FDA Approval for Andembry as a Prophylactic Treatment of Hereditary Angioedema (HAE)
Read More: CSL
Bayer Reports the US FDA’s NDA Submission of Gadoquatrane for Contrast-Enhanced MRI
Read More: Bayer
Gilead Reports the US FDA’s Approval of Yeztugo (Lenacapavir) for Pre-Exposure Prophylaxis (PrEP) to Prevent HIV in Individuals at Risk
Read More: Gilead
Incyte’s Monjuvi Regimen Receives the US FDA Approval for R/R Follicular Lymphoma
Read More: Incyte
Novartis Reports the Health Canada’s Approval of Kisqali (Ribociclib) for the Treatment of HR+/HER2- Early Breast Cancer
Read More: Novartis
ExCellThera Reports CHMP’s Positive Opinion for Zemcelpro to Treat Haematological Malignancies
Read More: ExCellThera
Regeneron and Sanofi Receive the US FDA’s Approval for Dupixent to Treat Bullous Pemphigoid
Read More: Regeneron and Sanofi
GSK Reports the MHLW’s sNDA Acceptance of Arexvy to Prevent Respiratory Syncytial Virus (RSV) Disease
Read More: GSK

AstraZeneca Partners with CSPC Pharmaceuticals to Identify and Develop Small Molecule Across Chronic Indications
Read More: AstraZeneca and CSPC Pharmaceuticals
NextCure Signs a ~$745M Licensing Agreement with Simcere Zaiming for SIM0505
Read More: NextCure and Simcere Zaiming
Teva Partners with Fosun Pharma to Accelerate Development of TEV-56278 in Immuno-Oncology
Read More: Teva and Fosun Pharma
Avata Biosciences Enters a Co-Development and Licensing Deal with Oceanus Bio for AVAT-021 and AVAT-022 to Treat Epilepsy and Schizophrenia
Read More: Avata Biosciences and Oceanus Bio
Processa Pharmaceuticals and Intact Therapeutics Enter Binding Term Sheet for PCS12852 Licensing Deal
Read More: Processa Pharmaceuticals and Intact Therapeutics

Inquis Medical Reports the US FDA’s 510(k) Clearance of AVENTUS Thrombectomy System to Treat Pulmonary Embolism
Read More: Inquis Medical
Brain Navi Biotechnology Reports the US FDA’s 510(k) Clearance of NaoTrac for Neurosurgical Procedures
Read More: Brain Navi Biotechnology
Neuspera Medical’s Integrated Sacral Neuromodulation System Secures the US FDA Approval for Urinary Urge Incontinence
Read More: Neuspera Medical
Johnson & Johnson MedTech Reports the US Launch of VOLT Wrist and Proximal Humerus Plating Systems
Read More: J&J MedTech

Supernus Pharmaceuticals to Acquire Sage Therapeutics for ~$795M
Read More: Supernus Pharmaceuticals and Sage Therapeutics
Eli Lilly to Acquire Verve Therapeutics for ~$1.3B
Read More: Eli Lilly and Verve Therapeutics

The US FDA Approves Additional Presentation of Celltrion’s Steqeyma (Biosimilar, Stelara)
Read More: Celltrion

ELIAS Animal Health Reports Interim Study Data of ELIAS Cancer Immunotherapy (ECI) in Canine Osteosarcoma
Read More: ELIAS Animal Health

Bracco Imaging & Subtle Medical’s AiMIFY Receives the European CE Mark Approval for Enhanced Brain MRI
Read More: Bracco Imaging and Subtle Medical
Related Post: PharmaShots Weekly Snapshots (Jun 09, 2025 – Jun 13, 2025)

