Robotic Process Automation in Pharmaceutical Industry (RPA in Pharma)
Shots:
- Efficient, time-savvy, and promising, automation helps companies navigate the unexplored areas of businesses and unlocks the door to new opportunities. PharmaShots brings this month an informative take on RPA in the Pharmaceutical Industry with this article
- RPA bots aim to automate repetitive tasks and reduce human involvement in less productive work to boost efficiency. Pharmaceutical companies leverage the use of intelligent automation, including RPA, AI, and ML to enhance productivity, understand the dynamics of the market to outperform and mitigate the risks and losses
- Dive deep into the application of RPA in the pharma industry with PharmaShots enlightening article that explores the bright prospects that RPA offers while addressing its downsides, navigates the back-office optimization that comes with RPA, and unveils an efficacious IADII model to RPA implementation by Octavus Consulting
Time-consuming and loss-inducing traditional practices have no place in today’s fast-paced world. By infusing innovations and technological advances, industries have benefitted immensely. In the highly regulated and compliance-driven pharma industry, integrating technologies can be efficacious and challenging at the same time. Over the years, new-age technological advances have colossally impacted production, operations, and customer relations in various sectors. With cutting-edge technology expediting nuanced aspects of the industry now and then, Robotic Process Automation (RPA) has significantly impacted the operations in the pharmaceutical industry. Robotic process automation (RPA) in the pharmaceutical industry helps carry out extensive and time-consuming tasks easily and conveniently without disrupting the existing systems. The article unveils an introduction to RPA in the pharma industry and navigates its positives and downsides. The article explores back-office optimization through RPA bots followed by a potent implementation model by Octavus Consulting and an optimistic conclusion.
RPA, a Technological Trend in the Pharma Industry
RPA is a business process automation that leverages software robotics to automate repetitive tasks and reduce human involvement in less productive work to boost efficiency. Apart from the pharmaceutical industry, RPA is used profusely in banking, e-commerce, social media marketing, mortgage, and lending processes among others. By integrating intelligent automation in the pharmaceutical industry, companies have extraordinarily benefitted, when it comes to managing procurement and supply chain, drug development, clinical trials, and marketing. RPA helps pharma companies reduce costs, stick to the regulatory requirements, and produce and deliver drugs in the market that are safe, effective, and profitable in the long run.
Exploring the Bright Sides of RPA in Pharma
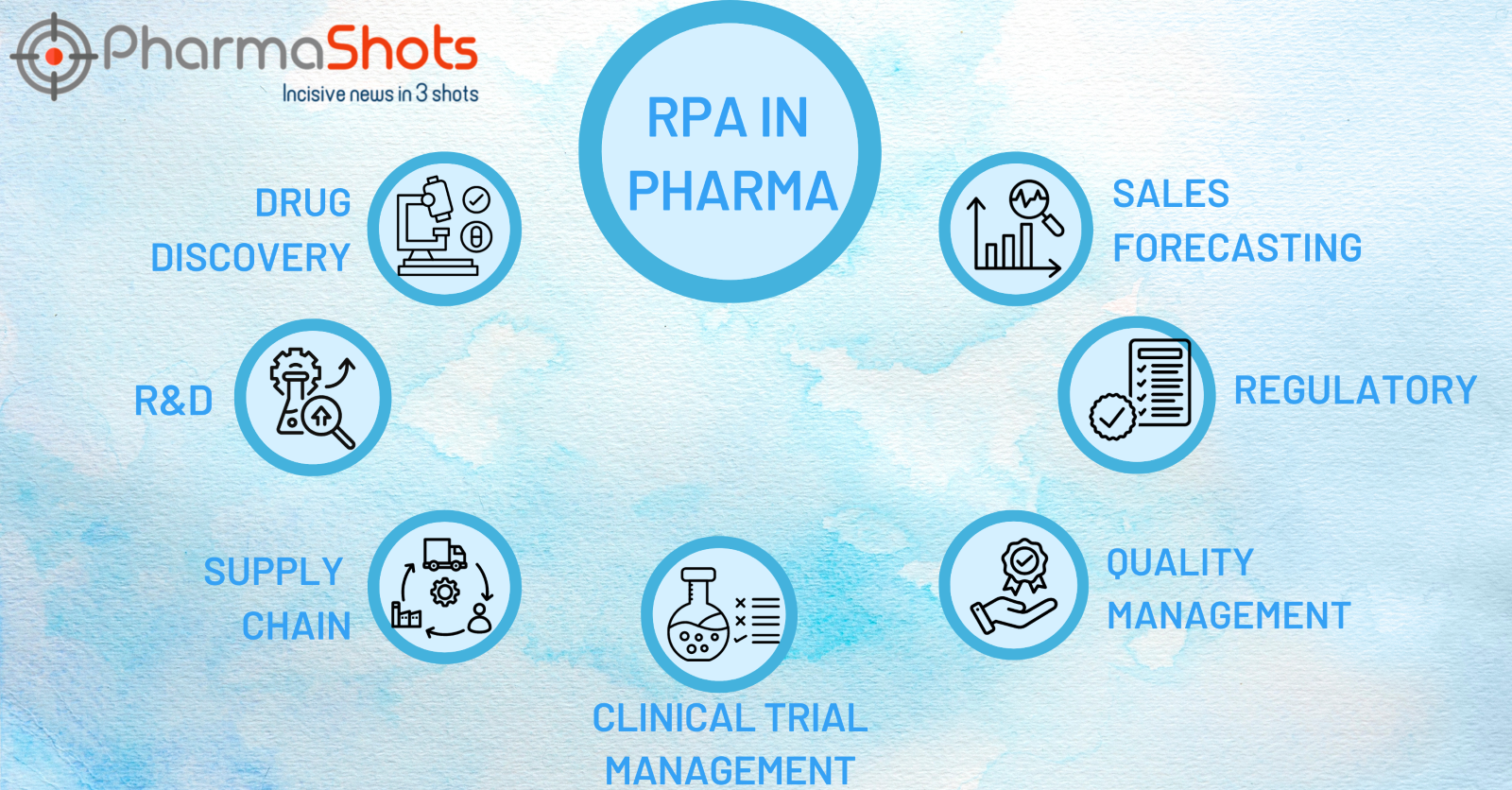
- Drug Discovery: With intelligent automation, the drug discovery process gets accelerated, thus saving a considerable amount of time, energy, resources, and manpower. Fueled with RPA, AI and ML help analyze data from virtually unlimited assays, expediting the identification of key drugs. Automation helps eliminate unrealistic candidates and provides time to focus on efficient molecules
- Research & Development: By fostering the use of intelligent automation, pharma companies save valuable time and resources wasted on evaluating assays and sample preparations
- Procurement and Supply Chain Management: RPA bots can help mitigate errors in order creation, delivery tracking, invoice generation, and inventory management
- Clinical Trial Management: RPA has transformed the ways of clinical trials by automating patient data entry, protocol creation, data collection, and processing, and report writing, which conventionally consumed considerable resources and time
- Quality Management: RPA has been used extensively in enhancing the standard of the products, ever since intelligent automation came into play. Be it label checking, barcodes, or compliance documents, RPA has rigorously blended in quality management in the pharma industry to save time and resources
- Regulatory Compliance: To meet regulatory requirements, pharma companies rely on RPA software as there is a negligible chance of error in deviation from the set program, thereby increasing accuracy.
- Drug Sales Forecasting: Automating data gathering, and data analysis gets easy. Using old sales records, RPA bots with data manipulation and prediction, forecast drug sales
The pharma industry has mostly embraced automation and the use of RPA in areas like claims management, documentation management, and others can be seen widely.
The downside of RPA in Pharma
- Structural Change & Attrition: RPA bots indubitably help reduce the workload by automating repetitive mundane tasks, allowing companies to cut costs. Automation is often disparaged for resulting in structural change in an organization and many cases leads to cutting down the workforce
- Problematic Transition: Integrating RPA bots may seem easy from an aerial view but requires extensive groundwork and an understanding of the processes and operations of a pharma company. If carried out without proper study and review may cause heightened problems during and after post-post-transitioning
Streamlining Back Office Operations in the Pharma Industry with RPA
Automation can be of great service in handling back-office operations, especially in the pharma industry. Let’s have a look at some operations that can be handy with the use of RPA:
- Administrative & Finance: RPA bots can be instrumental in facilitating payment, bank conciliation, and tax fillings
- Human Resources: By deploying automation via. RPA bots, companies can effectively manage employee’s expenses, carry out documentation, and manage data
- Scheduled Entries: RPA proves to be highly efficient while making scheduled entries. With effectively programmed RPA bots, scheduled entries can be made with negligible error
- Data transfer between Systems: In pharma companies, data is considered an invaluable asset and to effectively manage and transfer data with zero errors, pharma companies extensively use automation
- Support Calling: With a defined set of instructions, RPA bots do exceptionally well in bolstering customer interaction. The support call facility used by pharma giants leverages RPA bots to handle queries and administer complaints, saving both resources and human labor
RPA-Adoption Model by Octavus Consulting
Without proper groundwork and assessment of the company’s present operations and management, adopting an RPA model can be risky. Backed by data-driven decisions, Octavus Consulting holds the upper hand in analyzing pharma companies’ operations and finances to provide our clients with an efficacious roadmap to success. Octavus’ five-step summarized model on RPA adoption sheds light on the nuanced aspects of automation that must be taken into consideration. For a detailed report, reach out to us at connect@pharmashots.com or bd@octavusconsulting.com. Let’s unfold Octavus’ five-step IADII model for RPA adoption:

- Identify: The first and foremost step to implementing RPA in pharma companies involves identifying the key areas that require automation like supply chain, data management, and regulatory requirements, among others. By sorting out the aspects and areas that could use RPA bots, companies can implement a holistic approach to redefining operations
- Analyze: Post-singling out the areas that require automation comes analysis. This stage involves deploying a team of professionals to analyze the information collected in the previous step which becomes instrumental in developing and designing RPA bots in different areas
- Design: The step leverages the use of the core team involved in the earlier steps to design several RPA bots for different areas of the company. Here, the focus remains on mitigating the manual work and automating different operations and processes effectively
- Implement: It involves a unified endeavor of teams to execute the designed RPA bots in several areas. The step must test the nuanced aspect of operations followed by an extensive quality check before handing out the bots for the processes
- Improvise: The final step is what makes the RPA adoption model by Octavus Consulting more flexible. By having room for improvement and improvisation based on the needs of present and future operations, you devise a strong, flexible, and productive model for automation
Conclusions & Perspectives
By fostering new-age innovations and technological advances in the existing work models, companies can benefit immensely. With intelligent automation, pharma companies can navigate unexplored aspects of drug development, focus on unmet needs, and leverage predictive analysis to study epidemiology and climate-induced epidemics beforehand. Robotic process automation has significantly transformed the ways the industry operates. With RPA bots handling operations with fewer errors, there’s more room for us humans to focus on different aspects and areas.
PharmaShots is a platform where real-world and real-time news meets veritable and trusted sources. We specialize in catering to the life science, biotech, biopharma, MedTech, and animal health industries. Ranging from interviews, guest posts, and curated reports, to digital and content marketing solutions, PharmaShots epitomizes trust and integrity. Our parent company Octavus Consulting caters to the growing needs of the healthcare industry and specializes in competitive intelligence, market research, and business intelligence.
Related Post: Intelligent Automation in Pharmaceutical Industry (AI & ML)



