Insights+: EMA Marketing Authorization of New Drugs in March 2023
Shots:
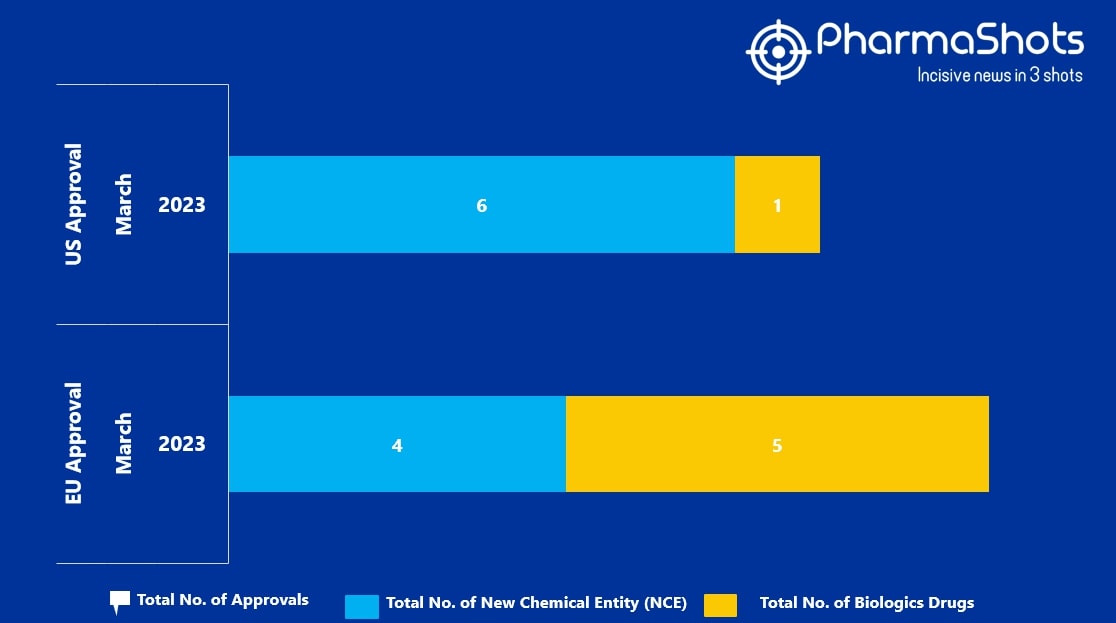
- The EMA approved 4 New Chemical Entity (NCE) and 5 Biologic Drugs in March 2023, leading to treatments for patients and advances in the healthcare industry
- In March 2023, the major highlights drugs were Reblozyl’s Approval for anemia in adult patients with non-transfusion-dependent beta thalassemia, Pombiliti for late-onset pompe disease
- PharmaShots has compiled a list of a total of 9 new drugs approved by the EMA in March 2023
1. Orion’s Darolutamide Receives EC’s Marketing Authorisation for the Treatment of Prostate Cancer
Nubeqa
Active ingredient: darolutamide Approved: March 01, 2023
Company: Orion Disease: Prostate Cancer
- The EC has granted marketing authorization for darolutamide + ADT in combination with docetaxel to treat patients with mHSPC
- The approval was based on the P-III trial (ARASENS) evaluating darolutamide (600mg, BID) + ADT and docetaxel vs ADT + docetaxel in a ratio (1:1) in 1306 patients with mHSPC. Darolutamide is developed jointly by Orion and Bayer
- The results showed a 32.5% reduction in risk of death with consistent benefits across clinically relevant 2EPs, the overall incidence of TEAEs was similar b/w treatment arms. Darolutamide was approved under the brand name “Nubeqa” in 80+ countries globally incl. the US, EU, Japan & China for nmCRPC & was also approved for mHSPC in a no. of markets incl. the US & Japan
Reblozyl
Active ingredient: luspatercept Approved: March 06, 2023
Company: BMS Disease: Non-Transfusion-Dependent Beta Thalassemia
- Reblozyl has received full marketing authorization from the EC to treat adult patients with anemia associated with non-transfusion-dependent beta-thalassemia. Reblozyl is being developed & commercialized through a collaboration with Merck
- The approval was based on the P-II study (BEYOND) evaluating Reblozyl vs PBO in a ratio (2:1) in 145 adults. The results showed 77.1% vs 0% of patients achieved the study’s 1EPs, ≥1.0 g/dL mean Hb increase from baseline
- In the 2EPs i.e., 49.0% vs 0% achieved a mean Hb increase of ≥1.5 g/dL over baseline from 37-48 wk. in the absence of transfusions, 89.6% vs 67.3% remained transfusion-free @1-24wk., improvements in patient-reported QoL outcomes were observed to correlate with Hb increases, serious AEs (11.5%)
Dupixent
Active ingredient: dupilumab Approved: March 21, 2023
Company: Regeneron Disease: Atopic Dermatitis
- The EC has approved Dupixent for sev. AD in children aged 6mos. to 5yrs. The approval was based on the P-III trial evaluating Dupixent (200/300mg, q4w) + low-potency TCS vs TCS alone in 162 children aged 6mos. to 5yrs.
- The results showed that 46% vs 7% of patients achieved ≥75% improvement in overall disease severity, 14% vs 2% achieved clear or almost clear skin & 55% vs 10% reduction in overall disease severity from baseline & 42% vs 1% reduction in itch from baseline
- Improvement in sleep quality, skin pain, and health-related QoL were seen in an overall and sev. populations while long-term efficacy data showed the clinical benefit at 16wks. sustained through 52wks. The safety results were consistent with the known safety profile of Dupixent
Hansizhuang
Active ingredient: serplulimab Approved: March 23, 2023
Company: Henlius Disease: Extensive-Stage Small Cell Lung Cancer
- The EMA has validated the application for Hansizhuang (serplulimab) in combination with CT (carboplatin and etoposide) for adults with ES-SCLC
- The application was based on the results from the P-III study (ASTRUM-005) evaluating the efficacy and adverse event profile of serplulimab + CT vs PBO + CT in 585 patients at 128 sites in multiple countries incl. China, Poland, Turkey, and Georgia. The results were presented at ASCO 2022 & were then published in the JAMA
- Hansizhuang, an anti-PD-1 mAb received ODD from the US FDA & EC for the treatment of SCLC. The therapy was also approved in China for the treatment of MSI-H solid tumors, sq. NSCLC and ES-SCLC
Pombiliti
Active ingredient: cipaglucosidase alfa Approved: March 27, 2023
Company: Amicus Therapeutics Disease: Late-Onset Pompe Disease
- The EC has approved Pombiliti (cipaglucosidase alfa) for the treatment of adults with late-onset Pompe disease (LOPD) used in combination with miglustat. The EMA’s CHMP opinion for miglustat is expected in Q2’23
- The approval was based on the results from the P-III (PROPEL) evaluating the efficacy, safety, and tolerability of Pombiliti
- Additionally, AT-GAA showed improvements in both musculoskeletal and respiratory measures in clinical studies. AT-GAA is a two-component investigational therapy that combines cipaglucosidase alfa to enable high-affinity uptake through the M6P receptor
Libtayo
Active ingredient: cemiplimab Approved: March 29, 2023
Company: Regeneron Disease: Non-Small Cell Lung Cancer
- The EC has approved Libtayo in combination with Pt-based CT for adult patients with advanced NSCLC with ≥1% PD-L1 expression & with no EGFR, ALK, or ROS1 aberrations
- The approval was based on the P-III trial (EMPOWER-Lung 3) evaluating Libtayo (350mg) + Pt-doublet CT vs Pt doublet CT alone in a ratio (2:1) in 466 patients which showed improvements across 1EPs & 2EPs incl. OS, 70% expressing PD-L1 ≥1%, m-OS (22 vs 13mos.) representing a 45% relative reduction in risk of death, m-PFS (9 vs 6mos.), ORR (48% vs 23%), m-DoR (16 vs 5mos.)
- Permanent treatment discontinuation due to AEs (5%) & the trial’s primary analysis results were published in Nature Medicine. Libtayo was approved in the EU & other countries for advanced BCC, CSCC, NSCLC & cervical cancer
Entresto
Active ingredient: sacubitril and valsartan Approved: March 31, 2023
Company: Novartis Disease: Heart Failure
- The EMA’s CHMP has adopted a positive opinion recommending approval of Entresto to treat symptomatic chronic HF with LV systolic dysfunction in pediatric patients aged 1-<18yrs.
- The opinion was based on the 52wk. results from the P-III trials (PANORAMA-HF) & (PARADIGM-HF) showed similar reductions from baseline in the cardiac biomarker NT-proBNP in adult & pediatric patients, numerically better improvements from baseline on a no. of clinically relevant EPs over enalapril
- The safety & tolerability were consistent with that observed in adult patients. If Entresto is approved, it will become available to children with a new age-appropriate formulation to enable accurate & convenient administration
Pedmarqsi
Active ingredient: sodium thiosulfate Approved: March 31, 2023
Company: Fennec Disease: Ototoxicity
- The EMA’s CHMP has issued a positive opinion recommending the marketing authorization of Pedmarqsi (sodium thiosulfate) in the US for the prevention of ototoxicity (hearing loss) induced by cisplatin CT in patients aged 1mos. to <18yrs. with localized, non-metastatic, solid tumors
- The opinion was based on the 2 P-III trials (SIOPEL 6) and (COG Protocol ACCL0431) evaluating Pedmarqsi + cisplatin-based regimen vs cisplatin-based regimens alone. The results from both studies showed that the hearing loss incidence rate was lower in the Pedmarqsi + cisplatin arm vs cisplatin alone (28.6% vs 56.4%) & (35.1% vs 67.3%)
- The EC will review the CHMP’s recommendation & issue a decision on Pedmarqsi’s approval by early June 2023. Pedmarqsi is currently marketed as Pedmark in the US
Briumvi
Active ingredient: ublituximab Approved: March 31, 2023
Company: TG Therapeutics Disease: Multiple Sclerosis
- The EMA’s CHMP has issued a positive opinion recommending the approval of Briumvi for adult patients with RMS with active disease defined by clinical or imaging features. The EC’s decision is expected in ~2mos.
- The opinion was based on the P-III trials (ULTIMATE I & II) evaluating Briumvi vs teriflunomide in 1094 patients across 10 countries. The trials were led by Lawrence Steinman, MD, Zimmermann Professor of Neurology & Neurological Sciences, and Pediatrics at Stanford University
- The results showed superiority over teriflunomide in reducing the ARR, the no. of T1 Gd-enhancing lesions & new or enlarging T2 lesions. The results were published in the NEJM. The centralized marketing authorization was valid in all EU MEMBER States, Iceland, Norway & Liechtenstein
Note: Entresto, Pedmarqsi & Briumvi received EMA’s CHMP Positive Opinion & EC’s Conditional Marketing Authorization for Darolutamide while EMA’s Validation of MAA for Hansizhuang (serplulimab)
Related Post: Insights+: EMA Marketing Authorization of New Drugs in February 2023





