EMA Marketing Authorization of New Drugs in October 2024
Shots:
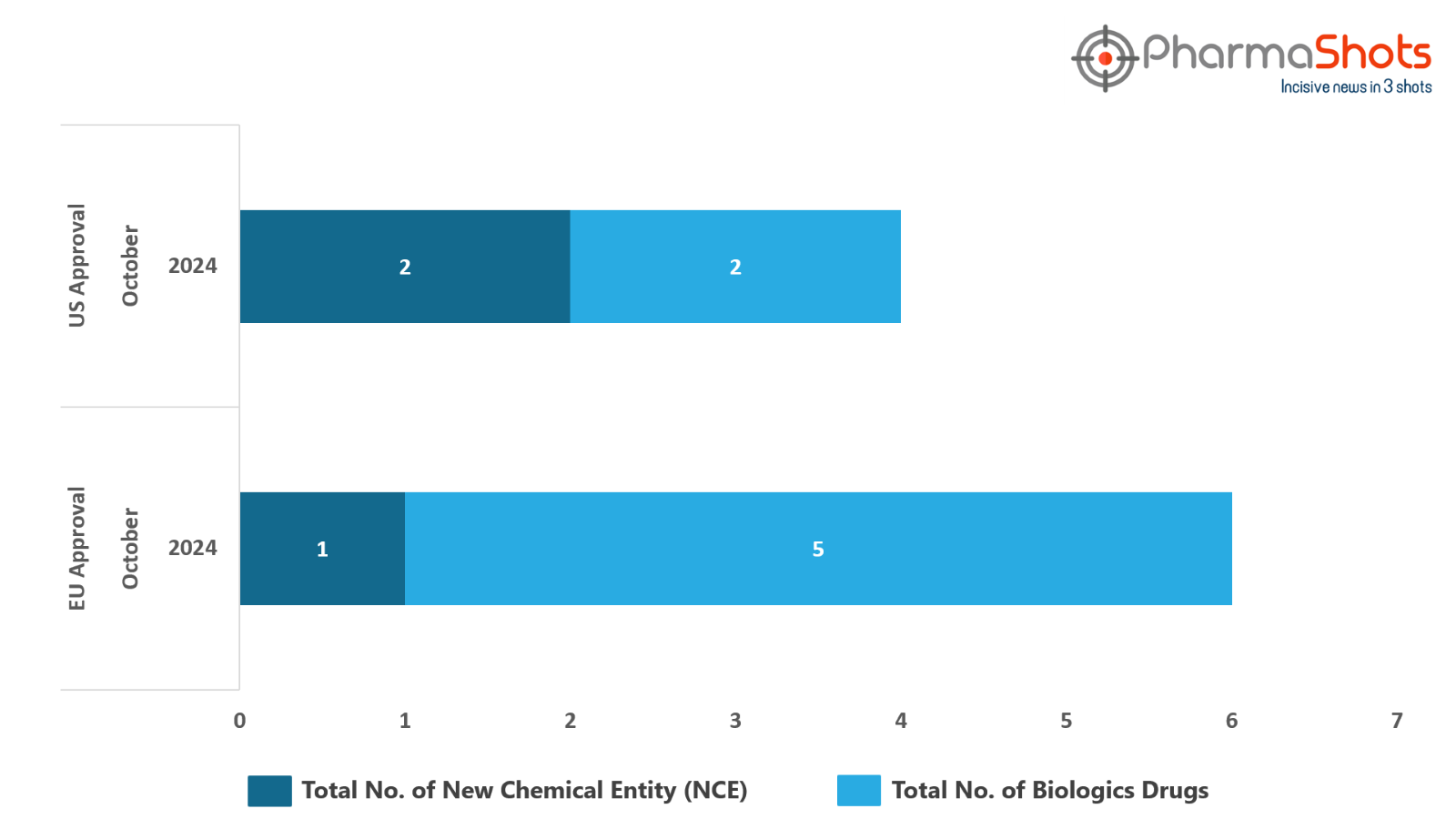
- The EMA’s CHMP has granted positive opinions to 5 Biologics and 1 New Chemical Entity in October 2024, leading to treatments for patients and advances in the healthcare industry
- The major highlighted drugs were Novo Nordisk’s Alhemo to treat Haemophilia A or B with inhibitors and AstraZeneca & Ionis’ Wainzua for Hereditary Transthyretin-Mediated Amyloidosis
- PharmaShots has compiled a list of 4 drugs that have been granted positive opinion by the EMA’s CHMP
Product Name: Wainzua
Active ingredient: Eplontersen
Company: AstraZeneca & Ionis
Date: Oct 17, 2024
Disease: Hereditary Transthyretin-Mediated Amyloidosis
Shots:
- The opinion of Wainzua for ATTRv-PN (stage 1/2 polyneuropathy) was supported by its P-III (NEURO-TTRansform) study vs external PBO for over wk.66, with a follow-up until wk.85 & an end-of-trial evaluation. Eligible patients could then enter an ongoing OLE study
- Study depicted sustained benefits in co-1EPs of serum transthyretin (TTR) levels & neuropathy impairment (mNIS+7) as well as 2EP of QoL (Norfolk QoL-DN) over 66wks., with a favorable & tolerable safety
- Eplontersen is under P-III (CARDIO-TTRansform) trial for transthyretin-mediated amyloid cardiomyopathy with 1,400 subjects. Both the companies are commercializing Wainzua for ATTRv-PN in the US & pursuing approval in the EU & ROW (AstraZeneca holds exclusive rights)
Product Name: Siiltibcy
Active ingredient: Mycobacterium tuberculosis derived antigens (rdESAT-6 and rCFP-10)
Company: Serum Institute of India
Date: Oct 17, 2024
Disease: Mycobacterium tuberculosis
Shots:
- The CHMP has recommended Siiltibcy (0.5μg/mL rdESAT-6 & rCFP-10) to diagnose Mycobacterium tuberculosis infection in individuals (age: ≥28 days), valid in the EU plus Norway, Iceland & Liechtenstein. Separate MAA will be submitted to the UK MHRA
- Siiltibcy’s sensitivity & specificity was assessed in comparison with QuantiFERON TB Gold In-Tube test (QFT, in-vitro test) & Tuberculin purified derivative (PPD RT23, an intradermal test)
- Serum Life Science Europe will be the MAA holder, Bilthoven Biologicals will handle import, release & commercialize Siiltibcy in the EU and Serum Institute of India will oversee manufacturing & regulatory compliance globally under an alliance b/w them
Product Name: Korjuny
Active ingredient: Catumaxomab
Company: Lindis Biotech
Date: Oct 17, 2024
Disease: Malignant Ascites
Shots:
- The CHMP has granted a positive opinion to Korjuny (trifunctional anti-CD3 x anti-EpCAM Ab) for treating malignant ascites in adults with EpCAM+ carcinomas, not eligible for systemic anti-cancer treatment. EC’s decision is anticipated by YE’24, & will be valid in the EU plus Norway, Iceland & Liechtenstein
- The opinion was based on P-II/III (IP-REM-AC-01) study showing four-fold increase in the 1EP of puncture-free survival vs therapy with only puncture treatment
- Moreover, recruitment for the P-I (CATUNIBLA) dose escalation & expansion study for high and intermediate-risk non-muscle invasive bladder cancer (HR-NMIBC) has been completed. Interim readouts were highlighted at the ESMO 2024
Product Name: Alhemo
Active ingredient: Concizumab
Company: Novo Nordisk
Date: Oct 17, 2024
Disease: Haemophilia A or B
Shots:
- The CHMP has recommended Alhemo (QD, SC) as a prophylactic treatment of hemophilia A/B with inhibitors in patients (≥12yrs.), with the EC’s decision anticipated within ~2mos.
- The opinion was based on the P-III (explorer7) study assessing the efficacy & safety of Alhemo to treat haemophilia A or B with inhibitors. Alhemo will be available in a convenient, pre-mixed, and prefilled pen upon approval
- Alhemo (concizumab) is a mAb that blocks anti-tissue factor pathway inhibitor (TFPI) for thrombin production, preventing blood clotting
Note: The following drugs have also been recommended for approval, however, no PR was available:
- Fluad
- Flucelvax
Related Post: Insights+: EMA Marketing Authorization of New Drugs in September 2024





