Insights+: The US FDA New Drug Approvals in August 2023
Shots:
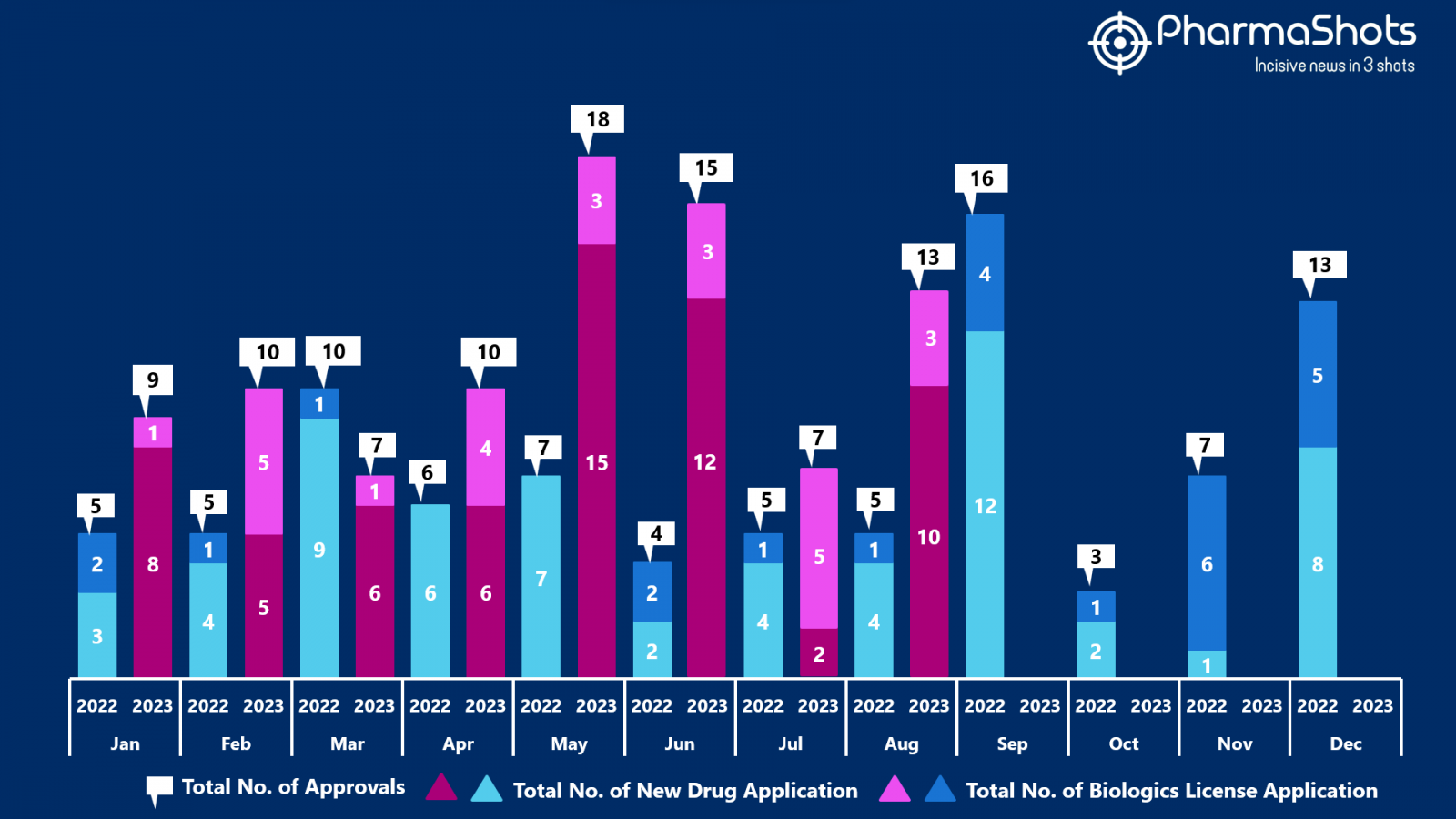
- The US FDA approved 10 NDAs and 3 BLA in August 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 89 novel products in 2023
- In August 2023, the major highlights drugs were Zurzuvae (zuranolone) approval for women with postpartum depression and Veopoz (pozelimab-bbfg) for children and adults with Chaple Disease
- PharmaShots has compiled a list of a total of 13 new drugs approved by the US FDA in August 2023
Lonsurf
Active ingredient: trifluridine/tipiracil Approved: Aug 03, 2023
Company: Taiho Oncology Disease: Metastatic Colorectal Cancer
- The US FDA has approved Lonsurf as monotx. or in combination with bevacizumab for mCRC before treated with fluoropyrimidine, oxaliplatin & irinotecan-based CT, an anti-VEGF biological therapy & RAS wild-type
- The approval was based on the P-III trial (SUNLIGHT) evaluating Lonsurf + bevacizumab vs Lonsurf alone in a ratio (1:1) in 492 patients., showed an improvement in OS & PFS, m-OS (10.8 vs 7.5mos.) with 39% reduction in risk of death, m-PFS (5.6 vs 2.4mos.) with a 56% relative risk reduction of disease progression
- Median time to worsening of the ECOG performance status score from 0 or 1 to 2 or more (9.3 vs 6.3mos.), OS & PFS benefits were associated with maintenance of QoL from baseline to cycle 6 with no clinical changes in mean scores in any subdomains for EORTC QLQ-C30 and EuroQol EQ-5D-5L HRQOL questionnaires & had a manageable safety profile
Zurzuvae
Active ingredient: zuranolone Approved: Aug 07, 2023
Company: Biogen and Sage Therapeutics Disease: Postpartum Depression
- The US FDA has approved Zurzuvae (50mg) in adults with PPD. The product is expected to be commercially available in Q4’23
- The approval was based on the results from the (NEST) clinical development program incl. two studies (ROBIN) and (SKYLARK) evaluating Zurzuvae vs PBO in adult women with PPD. Both studies met their 1EPs & showed a significant mean reduction from baseline in the 17-item HAMD-17 total score on Day 15
- The (SKYLARK) study met 2EPs with a significant reduction in depressive symptoms seen as early as Day 3 and sustained through Day 45, was well-tolerated with a consistent safety profile. The US FDA has issued a CRL for zuranolone’s NDA to treat MDD
Zurzuvae
Active ingredient: avacincaptad pegol Approved: Aug 07, 2023
Company: Iveric Bio Disease: Geographic Atrophy
- The US FDA has approved Izervay (complement C5 inhibitor) for the treatment of GA secondary to AMD. The therapy is expected to be available in the US in 2-4wks.
- The approval was based on the P-III trials (GATHER1 & 2) evaluating Izervay (2mg, qm, IVT) in 286 & 448 patients, which showed a significant reduction in the rate of GA progression over sham at 12mos. across two P-III clinical trials & slowed loss of photoreceptors and disease progression as early as 6mos. with ~35% reduction in 1yr. of treatment
- Additionally, the most common adverse reactions (≥ 5%) were reported at 12mos. across the (GATHER) clinical trial program in patients who received Izervay (2mg)
Akeega
Active ingredient: niraparib and abiraterone acetate Approved: Aug 11, 2023
Company: Janssen Disease: Prostate Cancer
- The approval was based on the P-III trial (MAGNITUDE) evaluating Akeega (200mg, qd) + prednisone vs PBO + abiraterone acetate + prednisone (AAP) in a ratio (1:1) in 765 patients showed a 47% risk reduction for rPFS
- At 2nd interim analysis with median follow-up at 24.8mos. in the BRCA+ subgroup, rPFS by central review showed a consistent trend favoring Akeega + prednisone with a m-rPFS (19.5 vs 10.9mos.), improvement in 2EPs of time to symptomatic progression (TSP) and time to initiation of cytotoxic chemotherapy (TCC) with an improvement in OS
- The safety profile was consistent with the known safety profile of each FDA-approved monotx., permanent discontinuation due to an adverse reaction was reported in 15% of patients
Daxxify
Active ingredient: botulinum toxin type A Approved: Aug 14, 2023
Company: Revance Therapeutics Disease: Cervical Dystonia
- The approval was based on the P-III program (ASPEN 1) & (ASPEN OLS) evaluating Daxxifyin incl. 382 patients and 1240 treatments across up to five inj. cycles over 88wk. Daxxify was shown to be effective in (ASPEN) study and was safe & well tolerated across both dose groups 125U and 250U with a median duration of effect of 24.0 & 20.3wks. for 2 dose groups
- In the (ASPEN OLS) study, symptoms continued to improve at doses ~300U, AEs remained low, and dysphagia rates (difficulty swallowing) remained low (2.7% for ASPEN-1) & (4.2% for ASPEN-OLS) thus supporting the safety profile of Daxxify
- Daxxify was approved in the US in Sept 2022 for adults with a temporary improvement of glabellar lines & received ODD for cervical dystonia in adults
Hepzato KIT
Active ingredient: Melphalan Approved: Aug 14, 2023
Company: Delcath Systems Disease: Hepatic-Dominant Metastatic Uveal Melanoma
- The US FDA has approved Hepzato KIT (melphalan/Hepatic Delivery System) as a liver-directed treatment for adult patients with mUM with unresectable hepatic metastases. The approval was based on the results from the P-III study (FOCUS) of Hepzato KIT via the hepatic delivery system (HDS) during a PHP procedure in 91 patients
- The results showed ORR was 36.3% in patients with hepatic and extrahepatic lesions and m-DoR was 14mos., DCR was 73.6% with 7 complete responses (7.7%), and PR (28.6%)
- The product is expected to be available in Q4’23 and enrolled patients will continue treated at Expanded Access Program (EAP) sites. Hepzato Kit is supplied with the Hepzato 5×5 Drug Pack and the HDS
Senvelgo
Active ingredient: velagliflozin Approved: Aug 16, 2023
Company: Boehringer Ingelheim Disease: Diabetes
- The US FDA has approved Senvelgo (velagliflozin oral solution), the first liquid once-daily, orally administered prescription medication to improve glycemic control in cats with diabetes mellitus
- Senvelgo oral solution, a highly selective inhibitor of the sodium-glucose co-transporter 2 (SGLT2) will be available in veterinary clinics in the US in mid-October. The product is also expected to be commercially available in countries globally
- By lowering elevated blood glucose levels and reducing the risk of clinical hypoglycemia events, Senvelgo oral solution improves the clinical signs of diabetes that cats suffer as soon as one week after starting treatment
Senvelgo
Active ingredient: palovarotene Approved: Aug 16, 2023
Company: Ipsen Disease: Fibrodysplasia Ossificans Progressiva
- The US FDA has approved Sohonos for adults & pediatric patients aged ≥8yrs. for females and ≥10yrs. for males with FOP. The capsules are indicated for the reduction in volume of new heterotopic ossification
- The approval was based on the P-III trial (MOVE) trial which showed a reduction in an annualized heterotopic ossification volume, a 54% reduction with a weighted linear mixed effect model. Palovarotene has a well-characterized safety profile with AEs consistent with the systemic retinoid class
- The 18mos. results were published in the Journal of Bone and Mineral Research. Ipsen Cares patient support program provides educational support, addresses coverage, access & reimbursement questions, also provides treatment access to eligible individuals in the US
Veopoz
Active ingredient: palovarotene Approved: Aug 18, 2023
Company: Regeneron Disease: Chaple Disease
- The US FDA has approved Veopoz for adult & pediatric patients aged ≥1yr. with CHAPLE disease. The approval was based on the P-II/III open-label trial of pozelimab
- Patients received a single loading dose of pozelimab (30mg/kg, IV on day 1), followed by weight-based doses SC, qw. The results showed normalization of serum albumin at 12wks. & maintained serum albumin concentrations through 72wks. of treatment, the median time for serum albumin to reach 3.5g/dL was reported to be 15.5days with a reduction in total no. of albumin transfusions & hospitalization days
- Veopoz is supplied as 400mg/2mL solution in a single-dose vial. Regeneron’s myRARE patient support program incl. assistance focused on the needs of eligible patients, incl. insurance coverage, financial support & information on treatment
Ingrezza
Active ingredient: valbenazine Approved: Aug 18, 2023
Company: Neurocrine Biosciences Disease: Chorea
- The approval was based on the 2 studies incl. the P-III study (KINECT-HD) & (KINECT-HD2) OLE trial evaluating Ingrezza vs PBO in 128 & 150 patients aged 18 to 75yrs. The (KINECT-HD) trial met its 1EPs of LSM change in chorea severity using the TMC score of UHDRS from the screening period baseline to the maintenance period (avg. of 10 & 12wks.) & showed an improvement in TMC score
- 4.6 vs 1.4-point improvement in chorea severity score from the start to the end of the 12wk. study. 40% reduction in chorea severity from baseline to maintenance & half of the patients saw a ≥40% reduction in HD chorea severity by 12wk.
- 43% & 53% were classified as “much improved” or “very much improved” on CGI-C & PGI-C vs 13% & 26% on PBO at 12wk. The company launched an INBRACE support program that provides access to patients to use Ingrezza & offers a patient assistance program to eligible patients
11. Regeneron’s Eylea HD (aflibercept) Receives the US FDA’s Approval for Serious Retinal Disease
Eylea HD
Active ingredient: aflibercept Approved: Aug 21, 2023
Company: Regeneron Disease: Serious Retinal Disease
- The US FDA has approved Eylea HD (8mg) for patients with wAMD, DME & DR. The recommended dose for Eylea HD is 8mg, q4w for the first 3 months across all indications, followed by 8mg, q8w-q16w in wAMD and DME & q8w-q12w for DR
- The approval was based on the 48wk. results from the (PULSAR) in wAMD & (PHOTON) trials in DME evaluating Eylea HD vs Eylea (2mg). Eylea HD is being jointly developed by Regeneron & Bayer AG
- Both trials met their 1EPs & showed non-inferior and clinical equivalent vision gains at 48wks. with 12 & 16wk. dosing regimens after 3 initial monthly doses vs 8wk. dosing regimen after initial monthly doses, the majority of patients were randomized at baseline to Eylea HD 12 or 16wk. dosing regimens were able to maintain dosing intervals through 48wks.
Abrysvo
Active ingredient: RSVpreF vaccine Approved: Aug 22, 2023
Company: Pfizer Disease: Respiratory Syncytial Virus
- The US FDA has approved Abrysvo (bivalent RSV prefusion F vaccine) for the prevention of LRTD & sev. LRTD caused by RSV in infants from birth up to 6mos. by active immunization of pregnant individuals at 32 through 36wks. gestational age.
- The approval was based on the P-III trial (MATISSE) evaluating the efficacy, safety & immunogenicity of Abrysvo in 7000+ pregnant individuals in a ratio (1:1) against LRTD & sev. LRTD due to RSV in infants born to healthy individuals vaccinated during pregnancy. The trial was conducted in 18 countries over 4 RSV seasons, followed by infants for ~2yrs.
- RSVpreF vaccine administered during pregnancy was effective against sev. RSV-associated lower respiratory tract illness in infants with no safety concerns, the incidence of AEs was similar b/w active & PBO groups in mothers and infants. The results were published in The NEJM
Reblozyl
Active ingredient: luspatercept Approved: Aug 29, 2023
Company: BMS Disease: Anemia
- The US FDA has approved Reblozyl for anemia without prior ESA-naïve use in adult patients with very low to intermediate-risk MDS who may require regular RBC transfusions. Reblozyl is being developed & commercialized through a collaboration with Merck
- The approval was based on interim results from a P-III trial (COMMANDS) evaluating Reblozyl vs epoetin alfa in 363 patients which demonstrated superior efficacy of concurrent RBC transfusion independence & Hb increase over epoetin alfa regardless of ring sideroblast status
- 58.5% vs 31.2% achieved the 1EPs of RBC-TI of 12wks. with concurrent mean Hb increase of 1.5g/dL within the first 24wks., HI-E increase (74.1% vs 51.3%) at 8wks., RBC-TI (47.6% vs 29.2%) within the first 24wks., RBC-TI (66.7% vs 46.1%) at 12wks. & showed durable responses with 2.5yrs. of median RBC-TI ≥12wks. The results were published in The Lancet
Related Post: Insights+: The US FDA New Drug Approvals in July 2023





