Molecular Farming: Paving a Modern Track for Pharmaceutical Sectors (Molecular Pharming)
Technologies and innovations have transformed the world globally. Traveling to different places, interacting with new people, working remotely from home, and much more comes with evolving technologies. With all these bright perspectives, it’s also an indisputable fact that we are now more susceptible to diseases than we were a few decades ago. After the outbreak of any epidemic or pandemic, all the responsibilities come on the shoulders of pharmaceutical companies. According to a survey, a majority from a cohort of pharmaceutical leaders expressed that the drug development plans, which ideally takes years of research, when asked to prepare in haste, may cause serious implications on the health of the population. With unprecedented diseases lurking on the corner, pharmaceutical companies, are now obligated to widen their conventional horizons and look for substitute practices in their drug development strategies. To overcome the requirement of large investment, running costs, poor scalability, risk of batch loss by contamination, and whose production is not possible by chemical synthesis, one common practice in the life science industry is molecular farming. This article covers molecular farming, its classification, and its role in the cancer treatment.
A Brief Introduction to Molecular Farming
Molecular farming is the practice of producing pharmaceutically significant and commercially valuable proteins or molecules in plants. Often referred to as “Pharming”, a portmanteau of pharmaceutical and farming, molecular farming is the production of recombinant pharmaceuticals outside their regular sources. It commences with the identification of a protein with desirable therapeutic or diagnostic activity followed by an analysis of protein or DNA sequencing, and finally, it is expressed in a heterologous host. Molecular farming unlocks virtually unlimited quantities of recombinant proteins for use as diagnostic and therapeutic tools in healthcare and life sciences industry. Even though molecular farming encompasses the production of any value-added protein like industrial enzymes, technical reagents, nutritional products, or protein-based materials, our emphasis in this article will primarily be on pharmaceutical proteins. Molecular farming dates back to 1980s, when the first recombinant human proteins were synthesized in the bacterium Escherichia coli. Since time immemorial, plants have been widely used as a source of medicinal compounds. Molecular pharming represents, a novel source of molecular medicines such as
- Plasma Proteins
- Proteins produced in plants for molecular farming can be categorized into four areas:
- Parental therapeutics and Pharmaceutical Intermediates
- Industrial Proteins
- Monoclonal Antibodies (mAbs)
- Antigens for Edible Vaccine
- Enzymes
- Growth Factors
- Vaccines
- Recombinant Antibodies
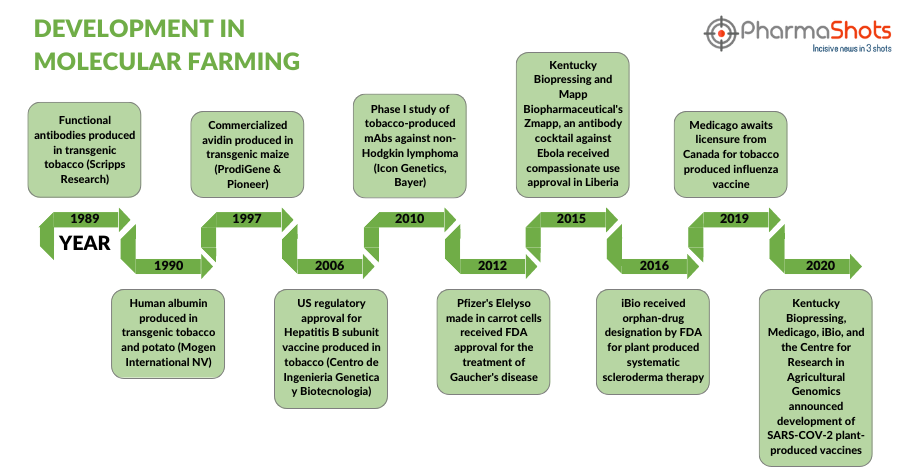
Timeline of Development in Molecular Farming
Over the years molecular farming has significantly molded the course of the pharmaceutical sector, which can be seen in this timeline
Advantages of Molecular Farming
Plant-based molecular farming offers substantial advantages for pharmaceutical sectors. Some of the key factors that give a significant upper hand to molecular farming are:
- Sustainability: Using plants as bioreactors is an environment-friendly choice, as plants are equipped with natural CO2 fixation, making them biodegradable.
- Safety: Molecular farming is a healthy alternative to other contemporary methods as there’re lower risks of batch loss, human contamination, and product cross-contamination.
- Scalability: It’s an irrefutable fact that molecular farming is highly scalable because of the significant amount of recombinant protein that can be produced from a single plant.
- Low Manufacturing Cost: With no prerequisite high-tech investments and maintenance, molecular farming has minimal requirements consequently making a huge impact on pharmaceutical sectors with cost cutting.
Limitations of Molecular Farming
Just like the two facets of the same coin, molecular farming comes with a few limitations too. Some of the decisive drawbacks that prevent companies to explore and unlock the possibilities of molecular farming are mentioned below:
- A low production level of proteins
- High rate of proteolysis
- Likely dispersal of transgenes to non-target organisms
Below mentioned Table 1, gives a brief insight into the plant species, biologic, and the condition or pathogen involved:

Table 1: Column 1 depicts the recombinant proteins manufactured from plant sources Column (2); Column 3 represents the disease conditions
Plant Molecular Pharming/Farming Production Platforms
The various parts of the plant that accumulate high levels of protein can be targeted are:
- Leafy crops: Due to its important biomass and high contents of soluble proteins, it can be used in the production of recombinant proteins ex. lettuce, alfalfa, and soybean
- Seeds: It reduce the need for fermenters and cold chains for storage and distribution, thus decreasing the overall costs of the production system Ex. cereals such as maize, barley, rice and wheat
- Non-food/non-feed crops: It avoid health issues to consumers present in food/feed crops Ex. tobacco
- Hairy roots: It result from natural transformation of plant cells with Agrobacterium rhizogenes (gram-ve bacteria). Different species of Solanaceae have capacities to produce heterologous proteins such as the E. coli B-subunit heat-labile toxin antigen
Common Method for Production of a Plant-based Vaccine, Developed from Virus-like Particles
- Gene Insertion: The microorganisms insert into plants (experimental host) that infect plants and transfer some of their genes to them Using Plant-Bioreactors to
- Synthesis: Once a virus has been genetically sequenced, various technologies (such as: Medicago’s Proficia) are used to synthesize the virus code that contains genetic instructions that plants can ‘read’, and insert it into bacteria that carries the information into the plant’s cells
- Infiltration: The plants are immersed in a liquid that contains a bacterium called Agrobacterium tumefaciens, which contains a sequence of a part of a virus gene. Using a vacuum, the air between the plant cells is sucked out. The plants then act like sponges, absorbing the liquid and the Agrobacterium tumefaciens that it contains
- Incubation: At the end, the plants are placed for at least four days in a friendly environment to grow. During this period the plants quickly produce large quantities of the most important ingredient, virus-like particles, or VLPs
- Harvest: After the growth, the plants are harvested, blending the leaves into a solution to extract the VLPs. However, these VLPs do not contain genetic material, making them non-infectious.
- Purification: VLPs are extracted from the leaves of mature plants and then purified to produce the final material that will be used for vaccines
Evolving Role of Molecular Farming in Cancer Treatment
Molecular farming is widely used in targeted therapies and immunotherapies, particularly in oncology, and has persuaded researchers all around the world to study the role of molecular farming in several therapy areas. The table below showcases a list of antibodies names, the plants they are derived from, and the applications

Table 2: Column 1 represents the recombinant proteins obtained from the plant sources (Column 2) with column 3 depicting the applications
Regulations for the Approval of Molecular Farming Products
Different jurisdictions follow distinct approaches toward the regulatory approval of molecular farming products. The focus areas in this article will be the US regulations and the EU regulations
- The US regulations focus more on the products than the process. The U.S. Code of Federal Regulations (CFR) Title 21 (Food and Drugs) covers molecular farming products and reagents including current good manufacturing practice (cGMP) covered by 21 CFR Parts 210211, good laboratory practice (GLP) toxicology (21 CFR 58), and a collection of good clinical practice (CGP) requirements specified by the ICH and accepted by the FDA5
- In the EU, molecular farming biopharmaceuticals are regulated by EMA and the local authority of the member state.
- These guidelines are largely specified by the European Commission (EC) in Directive 2001/83/EC and Regulation (EC) No 726/2004.
- However, upstream production in plants must also comply with additional laws relating to environmental release if grown outdoors (Directive 2001/ 18/EC, and 1829/2003/EC if the crop can be used as food/ feed) and Directive 2009/41EC if grown in constraint.
- Unlike the US, the EU has not yet approved transient expression for commercial development.
List of Some of the Leading Players, Contributing in Production of various Biopharmaceuticals with Molecular Farming
1. Transactiva srl, having the IP protection for the technology of molecular farming to produce biopharmaceuticals incl. biological drugs in rice seeds.
- The companys’ rice_PMF platform has completed PoC studies for various protiens:
- human lysosomal acid beta-glucosidase (rice_rhGCase)
- human lysosomal acid alpha-glucosidase (rice_rhGAA)
- human lysosomal acid alpha-galactosidase (rice_rhGLA)
- anti-cancer monoclonal antibody
- anti-inflammatory monoclonal antibody
2. Bright Biotech, developing plant-based affordable recombinant proteins by using chloroplasts
3. Planet Biotechnology, produced the first “plantibody” (plant-made antibody) based therapeutic and preventative products through cultivation of genetically modified green plants. It also provides contract- based services for production of recombinant proteins in Nicotiana benthamiana
4. Medicago, has a technology called Proficia through which the company has developed Covifenz, a plant based VLP, recombinant, adjuvanted vaccine for COVID-19 in collaboration with GSK
5. Protalix, developed Elelyso/UPLYSO by using its ProCellEx platform that received approval in May 2012 from the US FDA and being used for the treatment of Type 1 Gaucher disease
6. Leaf Systems International, developed a SUPRAVEC technology for the rapid simultaneous production of multiple gene products antibodies, vaccines and complex natural products in a controlled and coordinated manner within the tissues of plants and it is working towards the development of a transient expression system to use in the EU
Conclusions and Perspectives
With a growing influence of seeking eco-friendly alternatives to producing drugs, molecular farming has indeed emerged as a viable option for pharmaceutical sectors. The higher efficacy of monoclonal antibodies has persuaded scientists and researchers to dig deep into the field of molecular farming. The revolutionary change anticipated from molecular farming is to cater to medicines and improve healthcare systems in developing countries where the effervescent need for medicines has baffled world organizations.
References:
2. National Library of Medicine
3. Springer
5. PubMed
6. Transactiva
7. Nature.com
8. Medicago
10. Protalix
12. Leaf Systems International
Related Post: Pharmacy In The Era Of Telehealth & PharmaShots Interview: Medicago’s Brian Ward Shares Insights on COVIFENZ, a Plant-Based COVID-19 Vaccine




