
Insights+: The US FDA New Drug Approvals in October 2022
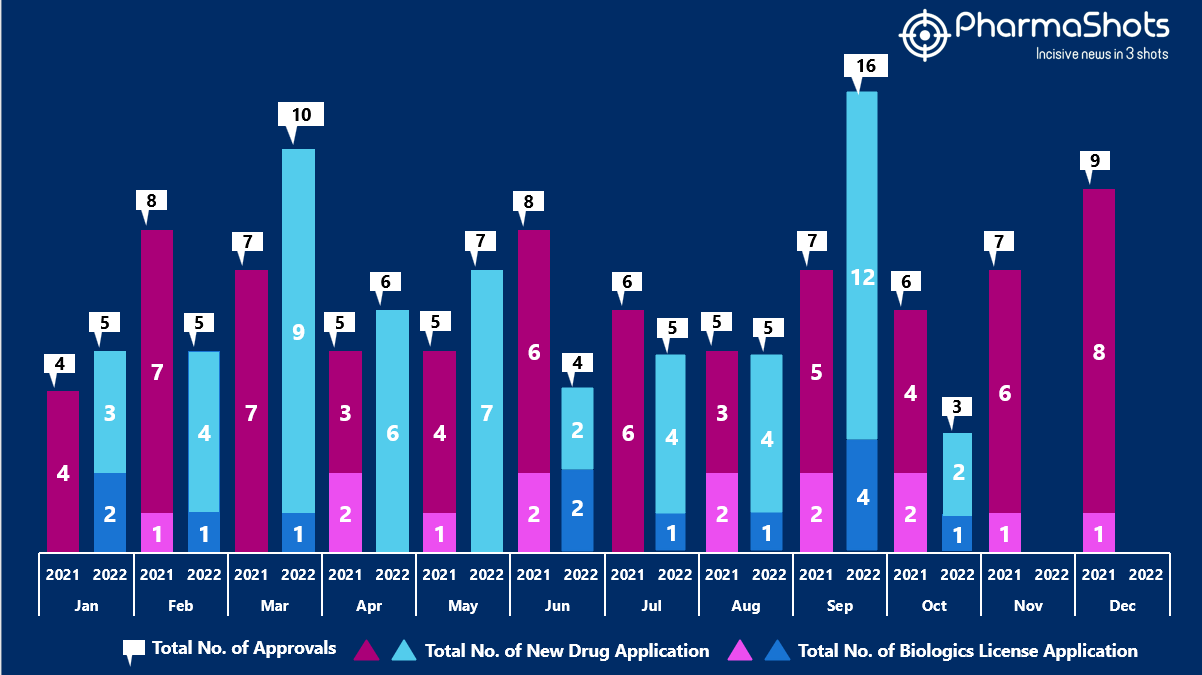
- The US FDA approved 1 NDAs and 2 BLA in October 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 66 novel products in 2022
- In October 2022, the major highlights drugs were Tecvayli’s approval for relapsed or refractory multiple myeloma, Furoscix for congestion in chronic heart failure
- PharmaShots has compiled a list of a total of 3 new drugs approved by the US FDA in October 2022
Furoscix
Active ingredient: furosemide Approved: October 10, 2022
Company: scPharmaceuticals Disease: Congestion in Chronic Heart Failure
- The US FDA has approved Furoscix (80mg/10mL) for congestion due to fluid overload in adults with NYHA Class II/III chronic HF
- Furoscix (SC infusion) demonstrated 99.6% bioavailability & 8hr. urine output of 2.7L similar to IV furosemide. The P-II (AT HOME-HF) pilot study evaluates furosemide (80mg/10mL) vs SoC in 51 patients with chronic HF with congestion uncontrolled by diuresis & showed a 37% lower risk of HF hospitalization @30 days & the 2EPs showed greater declines in mean patient body weight from baseline to day 2 & improvements in pulmonary-related metrics
- Furosemide is not indicated for use in emergency situations or in patients with APE. The formulation of furosemide is administered via On-Body Infusor & the product is expected to launch in Q1’23
Imjudo
Active ingredient: tremelimumab Approved: October 25, 2022
Company: AstraZeneca Disease: Liver Cancer
- The approval was based on the P-III (HIMALAYA) trial evaluating Imjudo (300mg) + Imfinzi (1500mg, q4w) vs sorafenib in 1324 patients with HCC prior not treated with systemic therapy & not eligible for LRT at 181 centers across 16 countries
- The results showed that patients treated with the combination experienced a 22% reduction in risk of death, 31% vs 20% were still alive after 3yrs. vs the same duration of follow-up. The results were published in the NEJM with no increase in severe liver toxicity or bleeding risk
- The safety profiles were consistent with the known therapy profiles with no new safety signals. The regulatory applications are currently under review in the EU, Japan & multiple other countries for advanced liver cancer
Tecvayli
Active ingredient: teclistamab Approved: October 27, 2022
Company: Janssen Pharmaceuticals Disease: Multiple Myeloma
- The US FDA has approved Tecvayli (teclistamab-cqyv) for the treatment of adult patients with r/r MM prior received ≥4 prior lines of therapy including a proteasome inhibitor, immunomodulatory drug, and anti-CD38 mAb
- In the P-II (MajesTEC-1) trial, the therapy showed ORR (61.8%) with CR (28.2%), and the median time to first response was 1.2mos. At a median follow-up of 7.4mos., the estimated DoR rate was 90.6% @6mos.and 66.5% @9mos., 78% received ≥4 prior lines of therapy, 76% were triple-class refractory
- Tecvayli is an off-the-shelf, SC therapy for patients with incurable blood cancer with limited treatment options. The product is supplied as 30mg/3mL and 153mg/1.7mL single-dose vials
Related Post: Insights+: The US FDA New Drug Approvals in September 2022

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














