Insights+: The US FDA New Drug Approvals in September 2022
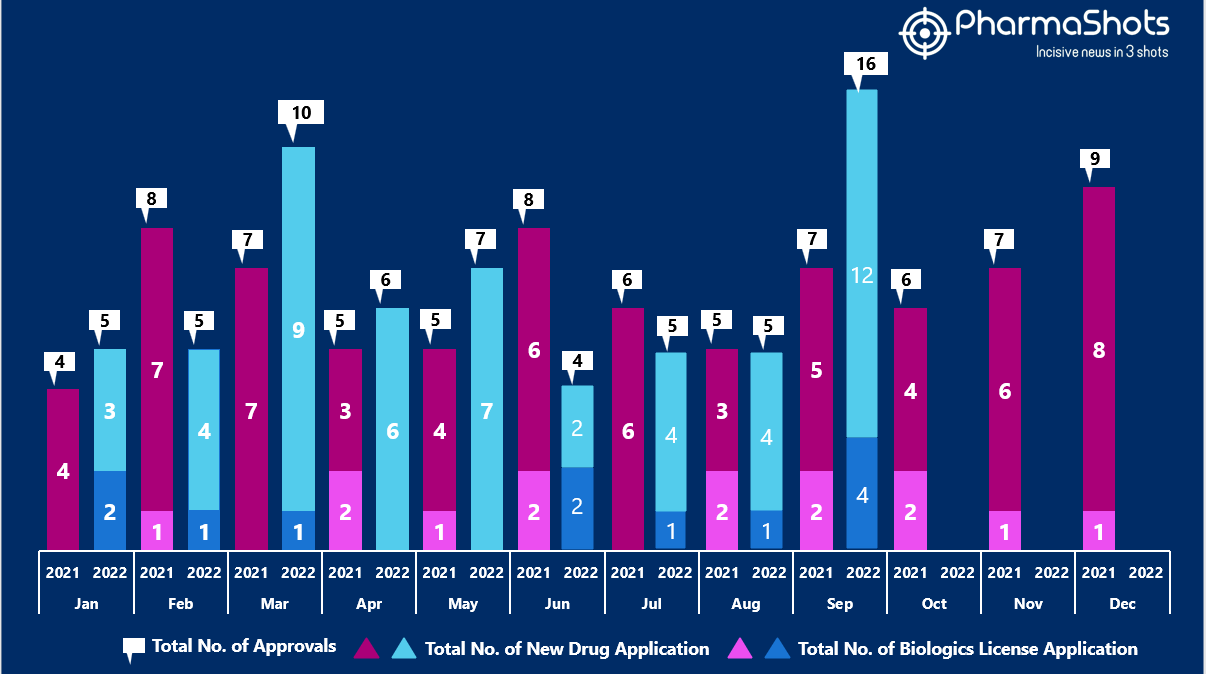
- The US FDA approved 12 NDAs and 4 BLA in September 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 63 novel products in 2022
- In September 2022, the major highlights drugs were Spevigo’s approval for generalized pustular psoriasis flares, Imfinzi for advanced biliary tract cancer, Retevmo for solid tumors with a RET gene fusion
- PharmaShots has compiled a list of a total of 16 new drugs approved by the US FDA in September 2022
Spevigo
Active ingredient: spesolimab-sbzo Approved: September 1, 2022
Company: Boehringer Disease: Generalized Pustular Psoriasis Flares
- The approval was based on the P-II (EFFISAYIL) trial evaluating Spevigo vs PBO in 53 patients with GPP flares
- The 12wk. P-II (EFFISAYIL) trial results showed that patients experienced a GPP flare, 54% vs 6% of patients treated with Spevigo for IV use showed no visible pustules one week after receiving treatment, AEs were reported in 66% vs 56%, serious AEs (6%), 17% and 6% had infections after 1wk.
- Spesolimab is currently under review by other regulatory authorities & has received BTD in China, Taiwan, US. The therapy has also received priority review in China and the US; ODD in Australia, Korea, Switzerland, and the US; RPD & FTD in Taiwan for GPP flares
Azurity’s Konvomep Receives the US FDA’s Approval for the Treatment of Active Benign Gastric Ulcer
Konvomep
Active ingredient: spesolimab-sbzo Approved: September 2, 2022
Company: Azurity Disease: Active Benign Gastric Ulcer
- The US FDA has approved Konvomep (omeprazole and sodium bicarbonate for oral suspension) for the treatment of active benign gastric. The therapy is also indicated for lowering the upper gastrointestinal bleeding risk in critically ill patients
- Konvomep is expected to be commercially available in Q1’23 & may give patients who have difficulty swallowing pills or capsules. Konvomep contains omeprazole (2mg), a proton pump inhibitor, and sodium bicarbonate per mL (84mg)
- The product is available in 90/150/300mL bottles. In July 2022, Azurity’s Zonisade has been approved in the US for partial seizures in adults and pediatric epilepsy patients aged ≥16yrs.
Imfinzi
Active ingredient: durvalumab Approved: September 5, 2022
Company: AstraZeneca Disease: Biliary Tract Cancer
- The approval was based on the P-III (TOPAZ-1) trial evaluating Imfinzi + CT vs PBO + CT in 685 patients with unresectable advanced or metastatic BTC at 105 centers across 17 countries incl. in the US, EU, South America & multiple countries in Asia incl. South Korea, Thailand, Japan & China
- In an interim analysis, Imfinzi + CT showed a 20% reduction in risk of death, 25% vs 10% were still alive @2yrs. & the results were consistent across all prespecified subgroups regardless of PD-L1 expression or tumor location. The results were presented at ASCO GI Symposium 2022 and published in NEJM
- The therapy was well tolerated and did not increase the discontinuation rate due to AEs. The regulatory applications are also under review in the EU & multiple other countries
Daxxify
Active ingredient: daxibotulinumtoxinA-lanm Approved: September 8, 2022
Company: Revance Disease: Glabellar Lines
- The US FDA has approved Daxxify for mod. to sev. frown lines (glabellar lines) in adults. The approval was based on the P-III (SAKURA) trial program i.e., (SAKURA 1/2/3) in 2700+ patients
- The results showed that 74% achieved a >2-grade improvement in glabellar lines @4wk. per investigator & patient assessment, 88% in >2-grade improvement as per investigator assessment, 98% with no or mild wrinkle severity with a median duration of 6mos. and maintained treatment results @9mos., significant improvement with long-lasting results and high patient satisfaction
- The therapy was proven to be effective, safe, and well tolerated with no serious TRAEs while the safety profile was consistent with other available neuromodulators
Rolvedon
Active ingredient: daxibotulinumtoxinA-lanm Approved: September 9, 2022
Company: Spectrum Pharmaceuticals Disease: Non-Myeloid Malignancies
- The approval was based on the P-III (ADVANCE) trial in 406 patients and (RECOVER) trials in 237 patients to evaluate eflapegrastim vs pegfilgrastim to reduce the risk of CT-induced neutropenia for breast cancer. The product is expected to be available in Q4’22
- The results from both trials showed that (15.8% vs 24.3% & 20.3% vs 23.5%) had sev. neutropenia during cycle 1, patients had toxicity lasting 1/2/3Days were 12% & 11%/3% & 8%/1% & 2% vs 15% & 17%/8% & 3%/1% & 3% while 42% reduction in mean duration of sev. neutropenia during cycle 1 in (ADVANCE) trial; relative risk reduction 8.5% vs 34.9%
- Mean duration of sev. neutropenia in cycle 1 was 0.20 ± 0.503 and 0.31 days vs 0.35 ± 0.683 and 0.39 days, AEs were consistent with prior results
Sotyktu
Active ingredient: deucravacitinib Approved: September 12, 2022
Company: Bristol-Myers Squibb Disease: Plaque Psoriasis
- The approval was based on the P-III (POETYK PSO-1 & 2) trials evaluating Sotyktu (6mg, qd) vs PBO & Otezla (30mg, BID) in 1684 patients aged ≥18yrs. with PsO. The therapy will be available in the US in Sept 2022
- The results showed superior efficacy & patients achieved PASI 75/PASI 90/sPGA 0/1 in (POETYK PSO-1) trials @16 & 24wks. (58% & 69% in Sotyktu vs 13% in PBO and 35% & 38% in Otezla/36% & 42% vs 4% and 20% & 22%/54% & 59% vs 7% and 32% & 31%) while (53% & 58% vs 9% and 40% & 38%/27% & 32% vs 3% and 18% & 20%/50% & 49% vs 9% and 34% & 30%) in (POETYK PSO-2) trial
- In the (POETYK PSO-1) trial, responses persisted through 52wk. who achieved PASI 75 @24wk. (82%) while in (POETYK PSO-2) trial, 80% vs 31%, AEs lead to discontinuation (2.4% vs 3.8% & 5.2%)
Terlivaz
Active ingredient: terlipressin Approved: September 15, 2022
Company: Mallinckrodt Disease: Hepatorenal Syndrome
- The US FDA has approved Terlivaz (terlipressin) in adults with hepatorenal syndrome (HRS) involving a rapid reduction in kidney function. The therapy is expected to be available in the US in the coming weeks
- The approval was based on the P-III (CONFIRM) trial to evaluate the safety and efficacy of terlipressin in patients with HRS-1 across the US and Canada
- The trial met its 1EPs of verified HRS reversal which was defined as renal function improvement, avoidance of dialysis, and short-term survival, patients had to be alive at least 10 days after attaining verified HRS reversal and not be receiving any renal replacement therapy during that time
Aponvie
Active ingredient: aprepitant Approved: September 20, 2022
Company: Heron Therapeutics Disease: Postoperative Nausea and Vomiting
- The US FDA has approved Aponvie injectable emulsion for IV use to prevent postoperative nausea & vomiting in adults
- The approval was based on the 2 clinical studies evaluating aprepitant vs IV ondansetron in patients with PONV. The results showed that aprepitant was more effective in preventing vomiting with ~50% fewer patients vomiting in the first 24 & 48hrs., after open abdominal surgery
- The P-I trial showed that 32mg of aprepitant as a 30-second IV injection was bioequivalent to aprepitant (40mg), was well tolerated with a comparable safety profile. The product is ready-to-use, easy to administer, innovative IV formulation & was supplied in a single-dose vial containing 32mg of aprepitant
Elucirem
Active ingredient: gadopiclenol Approved: September 20, 2022
Company: Guerbet Disease: Contrast-Enhanced Magnetic Resonance Imaging
- The US FDA has approved Elucirem for contrast-enhanced MRI
- The approval was based on the 2 P-III studies of gadopiclenol (0.05mmol/kg) vs gadobutrol (0.1mmol/kg) which demonstrated that gadopiclenol leads to non-inferior results in brain and body MRI, contrast enhancement & diagnostic efficacy at half of the gadolinium dosing of other GBCAs, no safety signals were reported & the adverse reactions were similar for both products
- Elucirem is a new macrocyclic gadolinium-based contrast agent with high relaxivity indicated for adults & children aged ≥2yrs. & can be utilized with MRI to detect lesions with abnormal vascularity in CNS & other areas. The product will be marketed by Guerbet in the US in a bottle and pre-filled syringe form
Fennec’s Pedmark Receives the US FDA’s Approval for the Treatment of Solid Tumors
Pedmark
Active ingredient: sodium thiosulfate Approved: September 21, 2022
Company: Fennec Disease: Solid Tumors
- The approval was based on the P-III (SIOPEL 6) & (COG ACCL0431) clinical trials evaluating the safety & efficacy data of Pedmark + cisplatin-based regimen vs cisplatin-based regimens alone in pediatric patients aged (>1mos.) with risk of cisplatin associated ototoxicity
- The result from the (SIOPEL 6) study demonstrated a consistent & significant reduction in hearing loss by 21.4% vs 73.3% whereas the (COG ACCL0431) study showed a reduction of 32.7% vs 63%. The study also showed a ≥85% increase in the 5yr. survival rate for patients
- Pedmark is formulated using sodium thiosulfate in single-dose vials for IV use in pediatric patients. Earlier, Pedmark has also received an ODD & a Priority Review designation from the US FDA in 2004
Retevmoi
Active ingredient: selpercatinib Approved: September 22, 2022
Company: Eli Lilly Disease: Solid Tumors
- The US FDA has approved Retevmo (40 & 80mg) for LA or metastatic solid tumors with RET gene fusion. Retevmo also received the traditional approval from the US FDA for LA or metastatic NSCLC with a RET gene fusion
- The 2 approvals were based on the P-I/II (LIBRETTO-001) trial for Retevmo. In RET fusion+ solid tumors, 90% received prior systemic therapy in patients with tumor-agnostic data, ORR (44%), CR (4.9%), PR (39%), m-DoR (24.5mos.) with 67% in ≥6mos. In NSCLC, ORR (84% in Retevmo vs 61% in CT), CR (5.8% vs 7.3%), PR (78% vs 54%), m-DoR (20.2 vs 28.6mos.), 50% vs 63% in ≥12mos.
- In NSCLC, 5 had measurable CNS metastases, 2 with RT to the brain within 2mos., 4 patients showed responses in intracranial lesions & 38% had an intracranial DoR of ≥12mos.
Omlonti
Active ingredient: Omidenepag isopropyl Approved: September 26, 2022
Company: Santen & UBE Disease: Glaucoma or Ocular Hypertension
- The approval was based on 3 clinical trials evaluating Omlonti (0.002%) in open-angle glaucoma or ocular hypertension patients with baseline IOP of ~24-26mm Hg for a duration of 3mos. The 3rd study included a 9mos. open-label treatment period following a 3mos. double-masked treatment period
- The results from the study indicated an IOP reduction for all the treatment arms incl. a reduction of IOP in the Omlonti arm from 5-7mm Hg across all 3 studies along with a reduction in timolol & latanoprost arm were 5-7 & 6-8mm Hg
- Omlonti is a relatively selective EP2 receptor agonist that reduces the elevated IOP by increasing aqueous humor drainage through the conventional/trabecular & uveoscleral outflow pathways
Iheezo
Active ingredient: chloroprocaine hydrochloride ophthalmic gel Approved: September 28, 2022
Company: Harrow and Sintetica Disease: Ocular Surface Anesthesia
- The US FDA has approved Iheezo (3%) for ocular surface anesthesia. The approval was based on the 3 P-III studies i.e., (Study 1 & 2) evaluated Iheezo in a ratio of (4:1) & (2:1) in 145 healthy volunteers, and (Study 3) evaluated Iheezo in patients undergoing cataract
- In (Study 1 & 2), 90% vs 12% & 95% vs 20% achieved anesthesia at a median time was 0.67 in both studies; the median duration of anesthesia was 14.3 & 19.3min. In (Study 3), patients achieved anesthesia in 1 to 1.5min., and no patients required supplemental treatment to complete the surgical procedure lasted on avg. 22min.
- Iheezo is supplied as a package of 1 or 10 units of 1.25mL single-patient-use vial containing 24mg of chloroprocaine in 800mg of gel. The product will available in May 2023
Dupixent
Active ingredient: dupilumab Approved: September 29, 2022
Company: Sanofi and Regeneron Disease: Prurigo Nodularis
- The US FDA has approved Dupixent for PN. The approval was based on the 2 P-III (PRIME) & (PRIME2) study evaluating Dupixent vs PBO in 311 adults with uncontrolled PN
- In both trials, 60% & 58% vs 18% & 20% of patients experienced a clinical reduction in itch from baseline @24wks.; 44% & 37% vs 16% & 22% @12wks., patients achieved clear or almost clear skin (48% & 45% vs 18% & 16%) @24wks.
- Additionally, 39% & 32% vs 9% & 9% experienced both clinical reductions in itch & clear or almost clear skin @24wks. & the safety results were consistent with the known safety profile in its approved dermatology indication. The regulatory filing is under EMA’s review for PN & submissions to regulatory authorities in additional countries are also planned in 2022
Lytgobi
Active ingredient: futibatinib Approved: September 30, 2022
Company: Taiho Disease: Intrahepatic Cholangiocarcinoma
- The US FDA has approved Lytgobi for adult patients with prior treated, unresectable, LA, or metastatic iCCA harboring FGFR2 gene fusions or other rearrangements. The therapy was discovered by Taiho Oncology’s parent company Taiho Pharmaceutical
- The approval was based on the results from the primary analysis of the P-II (FOENIX*-CCA2) trial evaluating Lytgobi (20mg, qd) in 103 patients The trial met its 1EPs & showed with ORR of 42% as measured by independent central review, m-DoR was 9.7mos. with 72% of responses lasting 6mos., was effective & well-tolerated
- The therapy covalently binds to FGFR2 and inhibits the signaling pathway while the other approved FGFR inhibitors are reversible ATP-competitive inhibitors
Relyvrio
Active ingredients: sodium phenylbutyrate and taurursodiol Approved: September 30, 2022
Company: Amylyx Disease: ALS
- The US FDA has approved Relyvrio (sodium phenylbutyrate and taurursodiol) for the treatment of adults with ALS
- The approval was based on the P-II (CENTAUR) OLE long-term follow-up phase trial to evaluate Relyvrio in 137 patients with ALS. The results showed a slowed loss of physical function & were published in the NEJM, Muscle & Nerve, and the Journal of Neurology, Neurosurgery & Psychiatry
- Relyvrio is an oral, fixed-dose combination therapy approved to treat adult patients with ALS in the US & approved as Albrioza for ALS in Canada. The MAA of AMX0035 is currently under the EMA’s review for ALS in the EU while the company also advanced the therapy for other neurodegenerative diseases
Related Post: Insights+: The US FDA New Drug Approvals in August 2022




