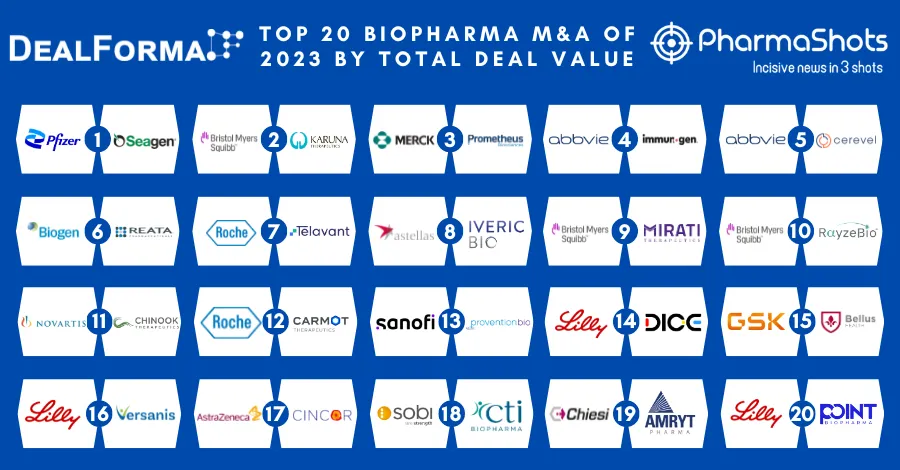
Amryt Publishes Results of Mycapssa in P-III (MPOWERED) Trial for the Treatment of Acromegaly in The Lancet Diabetes & Endocrinology
Shots:
- The P-III (MPOWERED) trial evaluates Mycapssa (oral octreotide capsules) vs iSRLs in patients with acromegaly. The trial is based on 2 P-III (CH-ACM-01) & (CHIASMA OPTIMAL) trial
- The results showed that Mycapssa was non-inferior to long-acting injectable octreotide or lanreotide in the maintenance of biochemical control, patients maintained response (91% vs 100%), mean IGF-1 was same at the start & end of the RCT phase in both groups
- Additionally, 15% vs 31% of patients reported breakthrough symptoms of acromegaly at the end of the RCT phase, reduction in no. of acromegaly symptoms. The trial helps to support the MAA for Mycapssa to the EMA which is currently under evaluation
Ref: Globe Newswire | Image: Globe Newswire
Click here to read the full press release

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com