Shots:
The company has introduced Uzpruvo, biosimilar of Stelara (ustekinumab) across the EU to treat gastroenterology, dermatology & rheumatology indications. Uzpruvo is available in a pre-filled syringes with a thinner needle & is latex-free to avoid allergic reactions
Further EU launches are expected in the upcoming mos., pending national price approvals through a fully European supply…

Shots:
The US FDA has approved Pyzchiva, a biosimilar of Stelara (ustekinumab), for treating moderate to severe plaque PsO patients eligible for phototherapy or systemic therapy, active PsA, moderate to severe active CD & moderate to severe active UC
Approval was based on studies of Pyzchiva vs Stelara incl. P-I, showing similarity in PK, safety, tolerability…

Shots:
The US FDA has approved Pyzchiva, a biosimilar of Stelara (ustekinumab), for treating moderate to severe plaque PsO patients eligible for phototherapy or systemic therapy, active PsA, moderate to severe active CD & moderate to severe active UC
Approval was based on studies of Pyzchiva vs Stelara incl. P-I, showing similarity in PK, safety, tolerability…

Shots:
The EC has granted marketing authorization for Pyzchiva (Biosimilar, Stelara) to treat autoimmune disorders in gastroenterology, dermatology, and rheumatology
The approval was based on P-I & P-III studies of Pyzchiva vs Stelara. P-I trial assessing the PK, safety, tolerability & immunogenicity in healthy volunteers, and P-III trial assessing the efficacy, safety & PK profile…
Shots :
Followed by the CHMP’s positive opinion in Nov 2023 for Crohn’s disease, psoriasis and psoriatic arthritis, the EC has approved Uzpruvo across the EU and Iceland, Liechtenstein, and Norway
The approval was based on the analytical & clinical results, data from the study (AVT04-GL-301) comparing the safety and efficacy of AVT04 vs Stelara…

Shots:
Stay up to date with PharmaShots Biosimilars Report, a monthly digest designed to keep you familiarized with the recent developments in biologics
Biosimilars are cost-effective alternatives to branded drugs but are very much alike when it comes to safety and efficacy
Check out the developments in the Biosimilars with PharmaShots’ illuminating report. PharmaShots has…

Shots :
The US FDA has accepted the BLA for DMB-3115 based on the results from P-III multi-regional studies for treating plaque psoriasis having rate of change in the Psoriasis Area and Severity Index (PASI) for skin symptoms as its 1EP. The results showed similar quality, safety and efficacy between DMB-3115 vs Stelara
Accord BioPharma…
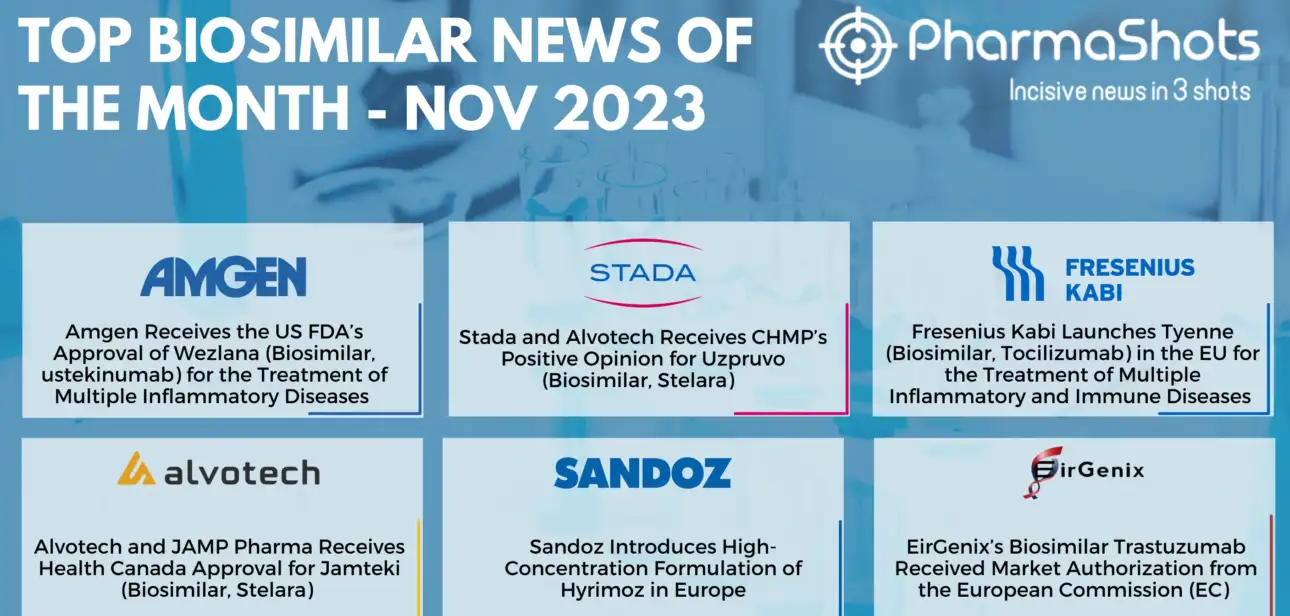
Shots:
Biosimilars are developed to be highly similar versions of approved biologics in terms of safety, purity, and potency
Biosimilars are expected to be a cost-effective alternative to the high-priced branded biologics, offering significant and much-needed cost savings to both payers and patients
During November, Amgen received US FDA approval for Wezlana, biosimilar of ustekinumab &…

Shots:
Prescription drugs have an instrumental role in effectively controlling chronic diseases like heart-related conditions, diabetes, arthritis, hypertension, and cancer, among others. Unlike over-the-counter drugs, prescription drugs are advised not to be taken without the authorization of a doctor
In 2022, the global market size of prescription drugs was worth $1035.5B and is envisioned to…

Biosimilars are developed to be highly similar versions of approved biologics in terms of safety, purity, and potency
Biosimilars are expected to be a cost-effective alternative to the high-priced branded biologics, offering significant and much-needed cost savings to both payers and patients
During the month of October, Boehringer launched adalimumab injection (biosimilar, Humira) for multiple…

