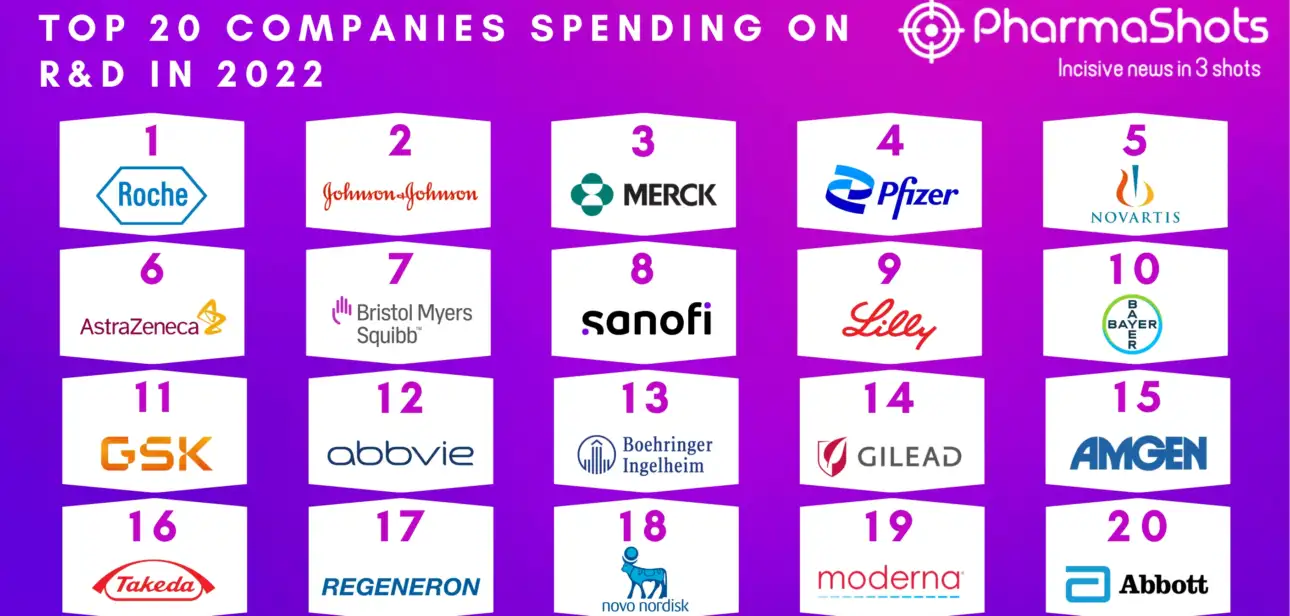
Shots:
Biocon, Bayer & Regeneron have signed an agreement to commercialize and distribute Yesafili, the biosimilar version of Eylea (aflibercept) Injections across Canada
Under the terms of the agreement, Biocon has set Jul 1, 2025, as the launch date for Yesafili (2mg NDS for vials and prefilled syringes). Earlier in Mar 2023, Health Canada approved…