
PharmaShots Weekly Snapshots (February 3, 2025 – February 7, 2025)
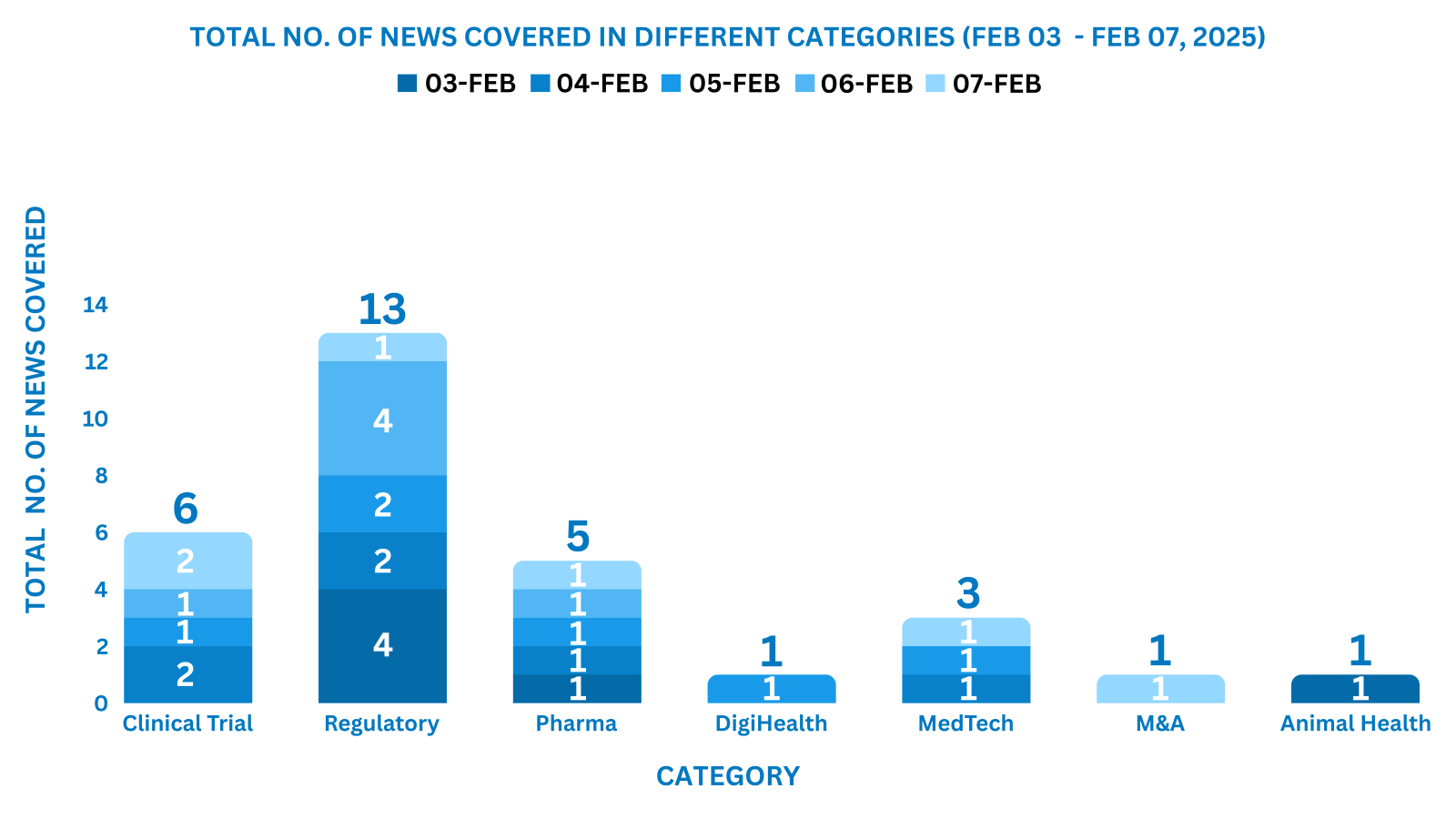
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Animal Health & DigiHealth. Check out our full report below:

Algiax Pharmaceuticals Reports Topline Data from P-IIa Trial of AP-325 for Neuropathic Pain
Read More: Algiax Pharmaceuticals
Pfizer Reveals Data from the P-III (BREAKWATER) trial of Braftovi Regimen in Patients with Metastatic Colorectal Cancer
Read More: Pfizer
Cumberland Pharmaceuticals Reveals Topline Data from P-II (FIGHT DMD) Trial of Ifetroban for Duchenne Muscular Dystrophy Heart Disease
Read More: Cumberland Pharmaceuticals
Akeso Completes Patient Enrollment in P-III (HARMONi-6/AK112-306) Trial of Ivonescimab for Squamous NSCLC
Read More: Akeso
Merck Initiates P-III (waveLINE-010) Trial of Zilovertamab Vedotin to Treat Previously Untreated Diffuse Large B-Cell Lymphoma (DLBCL)
Read More: Merck
Novo Nordisk Reveals Interim Data from P-III (FRONTIER3) Trial of Mim8 for Haemophilia A with or without Inhibitors
Read more: Novo Nordisk

AstraZeneca and Daiichi Sankyo Receive CHMP’s Positive Opinion for Datroway to Treat HR+/HER2- Breast Cancer
Read More: AstraZeneca and Daiichi Sankyo
Merck Receives CHMP’s Positive Opinion for Capvaxive (Pneumococcal 21-valent Conjugate Vaccine) for Invasive Pneumococcal Disease (IPD)
Read More: Merck
Novo Nordisk Reports the US FDA’s Approval for Ozempic to Reduce Risk of Kidney Disease Progression & Cardiovascular Death in Type 2 Diabetes & Chronic Kidney Disease (CKD) Adults
Read More: Novo Nordisk
Bavarian Nordic Receives CHMP’s Positive Opinion for Vimkunya to Prevent Chikungunya
Read More: Bavarian Nordic
Johnson & Johnson Receives the CHMP Positive Opinion on Label Extension to SC Rybrevant for the Treatment of Advanced EGFR-Mutated NSCLC
Read More: Johnson & Johnson
Bayer Submits MAA of Finerenone to the EMA for Heart Failure (HF)
Read More: Bayer
Roche Reports the US FDA’s Approval of Susvimo to Treat Diabetic Macular Edema (DME)
Read More: Roche
Japan’s MHLW Receives NDA for Conditional Approval of Astellas’ Avacincaptad Pegol to Treat Geographic Atrophy
Read More: Astellas
The US FDA grants RMAT Designation to Gradalis’ Vigil for Advanced Ovarian Cancer
Read More: Gradalis
Accord Healthcare (Intas) Receives EC Approval for Hetronifly (Serplulimab) as 1L Treatment of ES-SCLC
Read More: Accord Healthcare (Intas)
AstraZeneca Reports CHMP Positive Opinion for Imfinzi to Treat Limited-Stage Small Cell Lung Cancer (LS-SCLC)
Read More: AstraZeneca
Adicet Bio’s ADI-001 Secures the US FDA’s Fast-Track Designation for Treating Refractory SLE with Extrarenal Involvement
Read More: Adicet Bio
The US FDA Approves Supernus’ Onapgo (Apomorphine Hydrochloride) for Parkinson’s Disease
Read More: Supernus

Jupiter Neurosciences Collaborates with Zina Biopharmaceuticals to Advance Jotrol in P-II Trial for Parkinson’s Disease
Read More: Jupiter Neurosciences and Zina Biopharmaceuticals
Vanda Pharmaceuticals Enters into an Exclusive Global License Agreement with AnaptysBio to Develop and Commercialize Imsidolimab
Read More: Vanda Pharmaceuticals and AnaptysBio
Aldevron Collaborates with TriLink BioTechnologies for CleanCap mRNA Capping Technology
Read More: Aldevron and TriLink BioTechnologies
Imagene Partners with Tempus AI to Develop AI-Powered Diagnostics for NSCLC
Read More: Imagene and Tempus AI
Relmada Therapeutics Acquires Sepranolone from Asarina Pharma for ~$3.12M
Read More: Relmada Therapeutics and Asarina Pharma

Datar Cancer Genetics Launches Exacta AI to Offer Personalized Treatment Options for Cancer Patients
Read More: Datar Cancer Genetics
Gyder Surgical Reports the US FDA’s 510(k) Clearance for Gyder Hip System
Read More: Gyder Surgical
Senseonics Seeks the CE Mark Approval for Eversense 365 System
Read More: Senseonics

Alumis Signs a Definitive Merger Agreement with ACELYRIN to Develop Novel Therapies for Immune-mediated Diseases
Read More: Alumis and ACELYRIN

Anivive Lifesciences Receives $20M Funding from Leonid Capital Partners to Develop Novel Programs for Pets
Read More: Anivive Lifesciences and Leonid Capital Partners

Dawn Health & Novartis Introduce Cora BC to Support Breast Cancer Patients
Read More: Dawn Health & Novartis
Related Post: PharmaShots Weekly Snapshots (January 27, 2025 – January 31, 2025)
Tags

Ridhi is an avid secondary researcher who follows trends in the biopharmaceutical and healthcare sectors to curate engaging content for the global audience. She works as a news editor at PharmaShots and loves to read books and explore new destinations.














