
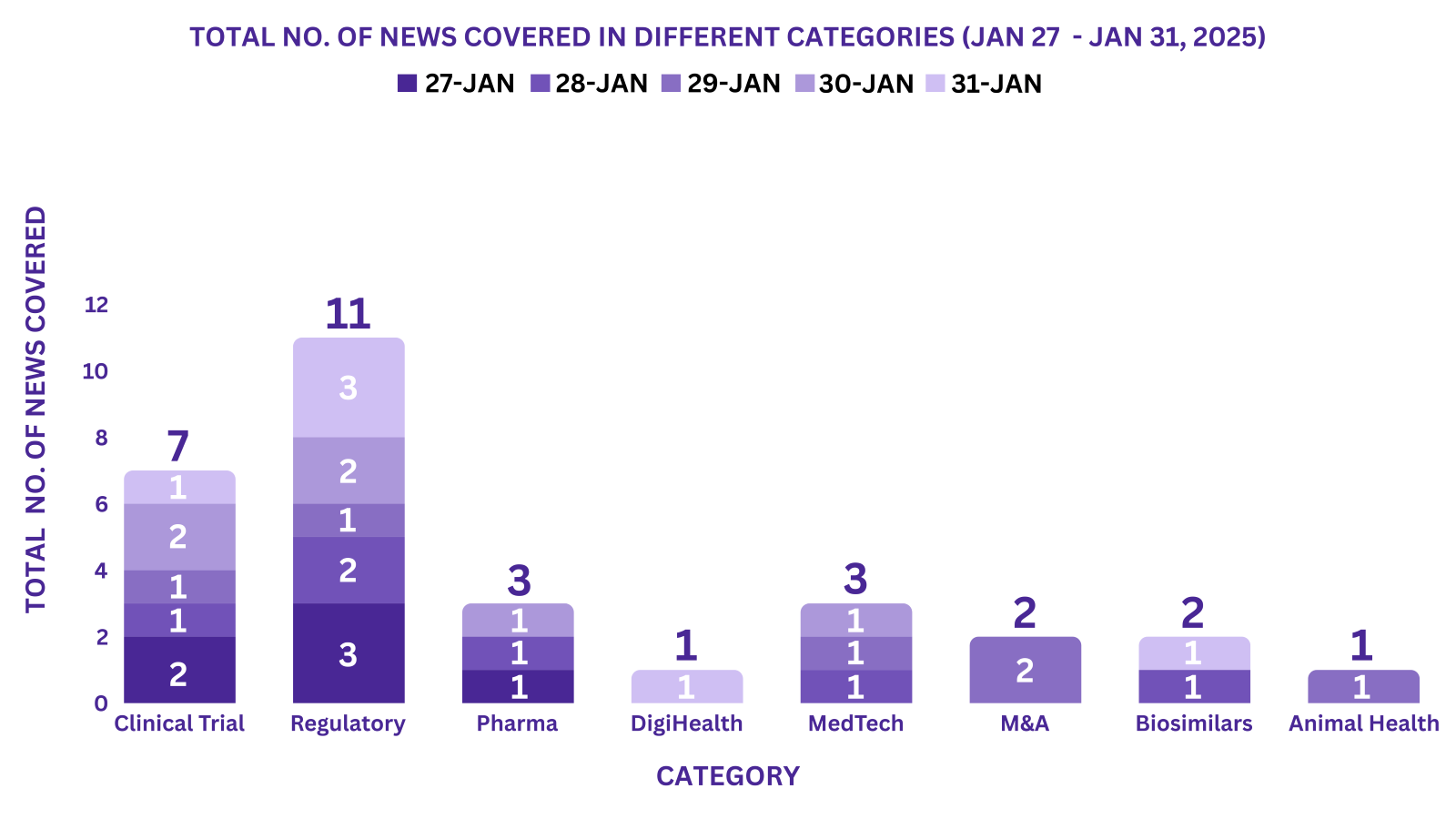
PharmaShots Weekly Snapshots (January 27, 2025 – January 31, 2025)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Biosimilars, Animal Health & DigiHealth. Check out our full report below:

Merck and Eisai Reveal Latest Data from P-III (LEAP-015) Trial of Keytruda (pembrolizumab) and Lenvima (lenvatinib) Regimen to Treat Gastroesophageal Adenocarcinoma
Read More: Merck and Eisai
Novo Nordisk Completes P-Ib/IIa Study of Subcutaneous Amycretin in Overweight or Obese People
Read More: Novo Nordisk
Roche Reveals Data from P-III (EMBARK) Study of Elevidys in Ambulatory Boys with Duchenne Muscular Dystrophy
Read More: Roche
ITM Reports Topline Data from P-III (COMPETE) Trial of ITM-11 to Treat Grade 1 or Grade 2 Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs)
Read More: ITM Isotope Technologies
Roche Reports Topline Data from P-III (INAVO120) Trial of Itovebi (Inavolisib) to Treat HR-Positive Breast Cancer
Read More: Roche
Ethris Reports Topline Data from P-I trial of ETH47 for Uncontrollable Asthma
Read More: Ethris
Daiichi Sankyo and AstraZeneca Dose the First Patient with Adjuvant Datroway in P-III (TROPION-Lung12) Trial for Adenocarcinoma NSCLC
Read More: Daiichi Sankyo and AstraZeneca

The US FDA Grants Fast Track Designation to Clarity’s Cu-64 SAR-BisPSMA in Biochemical Recurrence of Prostate Cancer
Read More: Clarity
China’s NMPA Accepts NDA for Akeso’s Gumokimab to Treat Moderate to Severe Psoriasis
Read More: Akeso Biopharma
Dyne Therapeutics’ DYNE-101 Secures the US FDA’s Fast Track Designation for Treating DM1
Read More: Dyne Therapeutics
Daiichi Sankyo and AstraZeneca Report the US FDA’s Approval of Enhertu for HER2 Low/Ultralow Metastatic Breast Cancer (MBC), Progressed on Endocrine Therapies
Read More: Daiichi Sankyo and AstraZeneca
The EMA Accepts MAA for GSK’s Depemokimab as an Adjunctive to Treat Asthma with Type 2 Inflammation and CRSwNP
Read More: GSK
The EMA Accepts MAA for ImmunityBio’s Anktiva to Treat BCG-Unresponsive Non-Muscle Invasive Bladder Cancer (NMIBC)
Read More: ImmunityBio
Amgen Receives EC’s Approval for Blincyto to Treat Philadelphia Chromosome-Negative CD19-Positive B-Cell Precursor Acute Lymphoblastic Leukemia (B-ALL)
Read More: Amgen
Scholar Rock Reports the US FDA's BLA Submission for Apitegromab to Treat Spinal Muscular Atrophy (SMA)
Read More: Scholar Rock
Axsome Therapeutics Receives the US FDA Approval for Symbravo (meloxicam and rizatriptan) to Treat Migraine
Read More: Axsome Therapeutics
ViiV Healthcare Reports the EC’s Approval of Vocabria + Rekambys to Treat HIV
Read More: ViiV Healthcare
Sanofi Receives China’s NMPA Approval for Sarclisa + Standard-of-Care VRd to Treat Newly Diagnosed Multiple Myeloma (NDMM)
Read More: Sanofi

Cstone Join Forces with SteinCares to Commercialize Sugemalimab in 10 Latin American Countries
Read More: Cstone Pharmaceuticals and SteinCares
Neurocrine Biosciences and Takeda Amend Partnership to Develop and Commercialize Osavampator in Japan
Read More: Neurocrine Biosciences and Takeda
Siolta Therapeutics Enters into a Joint Research Agreement with Cowellnex
Read More: Siolta Therapeutics and Cowellnex

ClearPoint Neuro Receives US FDA’s 510(k) clearance for ClearPoint Navigation Software Version 3.0
Read More: ClearPoint Neuro
Fresenius Kabi Reports the US FDA’s 510(k) Clearance for Adaptive Nomogram, Improving Plasma Collection Efficiency
Read More: Fresenius Kabi
Life Spine Receives US FDA’s 510(k) Clearance for ProLift Pivot Expandable Spacer System
Read More: Life Spine

Zimmer Biomet to Acquire Paragon 28 for ~$1.2B
Read More: Zimmer Biomet and Paragon 28
Lantheus Holdings to Acquire Evergreen Theragnostics for ~$1B
Read More: Lantheus Holdings and Evergreen Theragnostics

Alvotech & Teva Reports the US FDA’s BLA Acceptance of AVT05 (Biosimilar, Simponi & Simponi Aria)
Read More: Alvotech and Teva
Celltrion Secures the US FDA’s Approval for Avtozma (Biosimilar, Actemra)
Read More: Celltrion

Pet Honesty Introduces Urinary Tract Health for Cats
Read More: Pet Honesty

Bayer Introduces CanesMeno Digital Educational Hub and Products in the UK to Provide Menstrual Support
Read More: Bayer
Related Post: PharmaShots Weekly Snapshots (January 20, 2025 – January 24, 2025)
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














