
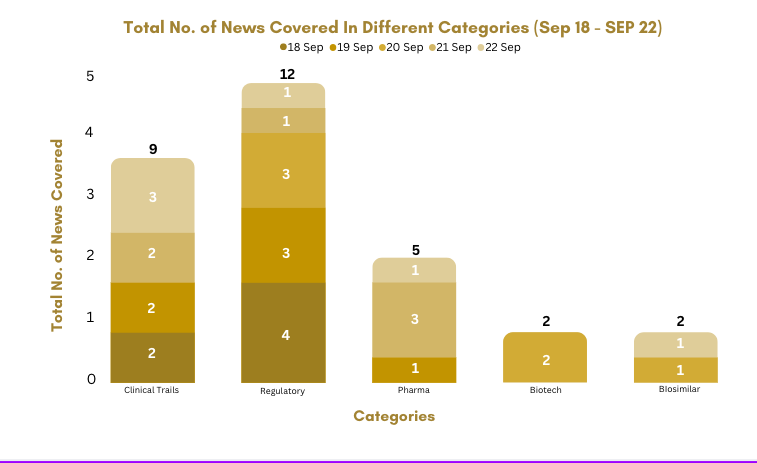
PharmaShots Weekly Snapshots (September 18–22, 2023)
This week PharmaShots’ news was all about the updates on clinical trials, regulatory, biotech, pharma, biosimilar. Check out our full report below:

- The EMA’s CHMP has adopted the positive opinion recommending the approval of AstraZeneca and Daiichi Sankyo’s Enhertu for HER2-mutant advanced NSCLC, was based on the P-II trial (DESTINY-Lung02)
Read more: AstraZeneca and Daiichi Sankyo
- The US FDA has approved GSK’ Ojjaara (momelotinib) for Myelofibrosis patients with Anemia, based on the P-III trial (MOMENTUM) & data for a subpopulation from the P-III (SIMPLIFY-1) trial
Read more: GSK
- The EMA’s CHMP recommended Merck’s Keytruda (pembrolizumab) as adjuvant treatment for NSCLC, based on results from the P-III trial (KEYNOTE-091)
Read more: Merck
- NICE has recommended Idorsia’s Quviviq (daridorexant) for adults with Chronic Insomnia
Read more: Idorsia
- The EC has approved Pfizer’s Litfulo (ritlecitinib) for adolescents and adults with severe alopecia areata, based on the (ALLEGRO) trial program incl. P-IIb/III trial (ALLEGRO)
Read more: Pfizer
- The EC has approved ViiV Healthcare’s Apretude (cabotegravir long-acting injectable and tablets) for HIV prevention, based on the results from two P-IIb/III active-controlled studies (HPTN 083 & 84)
Read more: ViiV Healthcare
- The US FDA’s has granted FTD to Kymera Therapeutics’ KT-333 for Cutaneous and Peripheral T-Cell Lymphoma
Read more: Kymera Therapeutics
- The US FDA has accepted the sNDA and granted Priority Review of Merck’s Welireg (belzutifan) for advanced renal cell carcinoma, based on the results from the P-III trial (LITESPARK-005)
Read more: Merck
- The NMPA has recommended a Priority Review for Everest Medicines’ cefepime-taniborbactam to treat Complicated Urinary Tract Infections
Read more: Everest Medicines
- The US FDA has accepted the sBLA and granted priority review for Merck’s Keytruda for newly diagnosed high-risk locally advanced cervical cancer, based on the P-III trial (KEYNOTE-A18/ENGOT-cx11/GOG-3047)
Read more: Merck
- The EC has approved Jazz’ Enrylaze for the treatment of acute lymphoblastic leukemia and lymphoblastic lymphoma, based on the P-II/III trial
Read more: Jazz
- Health Canada granted marketing approval to argenx’s Vyvgart for the treatment of Generalized Myasthenia Gravis (gMG)
Read more: argenx

- Kite highlighted P-II study (ALYCANTE) results of Yescarta (axicabtagene ciloleucel) for Relapsed/Refractory Large B-Cell Lymphoma showed a complete metabolic response
Read more: Kite
- Aclaris reported P-I (ATI-2138-PKPD-102) trial results of ATI-2138 for immuno-inflammatory diseases showed no serious AEs
Read more: Aclaris
- Iveric Bio highlighted 24-month results of Izervay (avacincaptad pegol) for Geographic Atrophy met the primary objective of reducing the rate of GA growth
Read more: Iveric Bio
- Rani Therapeutics reported an initiation of P-I study for RT-111 (biosimilar, ustekinumab) to treat Autoimmune Diseases
Read more: Rani Therapeutics
- Daiichi Sankyo and AstraZeneca highlighted P-III trial (TROPION-Breast01) results of Datopotamab Deruxtecan for Breast Cancer showed an improvement for the 1EPs of PFS
Read more: Daiichi Sankyo and AstraZeneca
- The US FDA granted Feedback to discontinue the development of Taysha Gene Therapies’ TSHA-120 program in giant axonal neuropathy
Read more: Taysha Gene Therapies
- Relmada Therapeutics, Inc reported results from the P-III clinical trial evaluating REL-1017 for major depressive disorders
Read more: Relmada Therapeutics
- Seagen and Astellas highlighted P-III Trial (EV-302) results of Padcev (enfortumab vedotin-ejfv) and Keytruda for advanced bladder cancer met its dual 1EPs of OS and PFS
Read more: Seagen and Astellas
BMS highlighted P-III trial (CheckMate -77T) results of N=neoadjuvant Opdivo (nivolumab) + CT for resectable non-small cell lung cancer met its 1EPs of improved EFS as assessed by BICR
Read more: BMS

- PeptiDream & Genentech collaborated to discover and develop novel peptide-radioisotope drug conjugates
Read more: PeptiDream & Genentech
- Exscientia & Merck KGaA collaborated to discover novel small molecule drug candidates
Read more: Exscientia & Merck KGaA

- The EC has approved Fresenius Kabi’s Tyenne (biosimilar, tocilizumab) for multiple inflammatory and immune mediated conditions
Read more: Fresenius Kabi
- Biocon Biologics’ Yesafili (biosimilar, aflibercept) Receives EC’s Approval for the treatment of patients with age-related macular degeneration and diabetic macular edema
Read more: Biocon Biologics

- Kezar Life Sciences & Everest Medicines collaborated to develop and commercialize Zetomipzomib for Lupus Nephritis
Read more: Kezar Life Sciences & Everest Medicines
- Inventiva enters into exclusive licensing agreement with Hepalys Pharma to develop and commercialize Lanifibranor in Japan and South Korea
Read more: Inventiva and Hepalys Pharma
- Evaxion Biotech A/S signed a collaboration agreement with Afrigen Biologics (Pty) Ltd to develop novel mRNA vaccines against Gonorrhea
Read more: Evaxion Biotech and Afrigen Biologics
- Orionis Biosciences and Genentech signed a multi-year collaboration to discover and develop molecular glue class medicines for the treatment of disorders including oncology and neurodegeneration
Read more: Orionis Biosciences and Genentech
- Boundless Bio & Taiho Oncology collaborated to evaluate Lytgobi + BBI-355 for FGFR amplified solid tumors
Read more: Boundless Bio & Taiho Oncology
Related Post: PharmaShots Weekly Snapshots (September 11–15, 2023)
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














