
PharmaShots Weekly Snapshots (September 11–15, 2023)
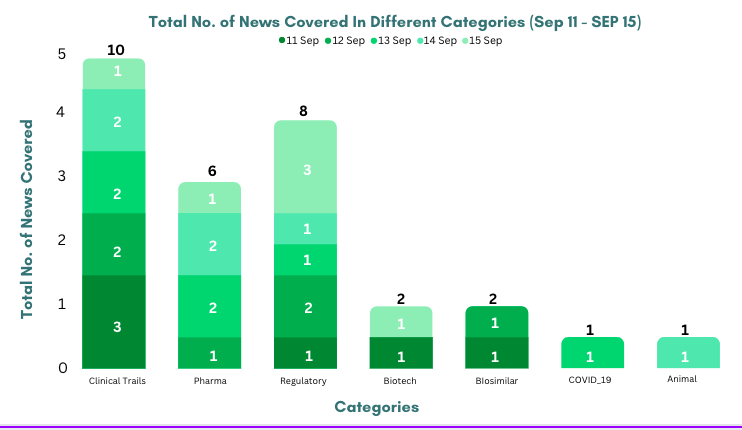
This week PharmaShots’ news was all about the updates on clinical trials, regulatory, biotech, pharma, biosimilar and Animal Health. Check out our full report below:

- AstraZeneca and Daiichi Sankyo highlighted initial results from the P-Ib (TROPION-Lung04) trial of datopotamab deruxtecan + Imfinzi as 1L advanced NSCLC showed an encouraging response with no new safety signals
Read more: AstraZeneca and Daiichi Sankyo
- BMS reported P-II study results of BMS-986278 for Progressive Pulmonary Fibrosis, presented at ERS 2023 showed 69% & 42% reduction in rate of decline in percent predicted forced vital capacity at 30 & 60mg doses
Read more: BMS
- Daiichi Sankyo reported P-II (HERTHENA-Lung01) trial results of Patritumab Deruxtecan for EGFR-mutated metastatic NSCLC, presented at WCLC 2023 showed clinically meaningful & durable responses
Read more: Daiichi Sankyo
- AstraZeneca highlighted P-III trial (FLAURA2) results of Tagrisso for EGFR-mutated advanced lung cancer showed an improvement in PFS & a 38% reduction in risk of disease progression or death
Read more: AstraZeneca
- Janssen highlighted P-Ib/II study (CHRYSALIS-2) results of Rybrevant (amivantamab-vmjw) + Lazertinib for EGFR-mutated advanced NSCLC showed durable progression-free survival, presented at IASLC 2023 WCLC
Read more: Janssen
- Hoth Therapeutics reports positive results of HT-KIT from preclinical study in Gastrointestinal Stromal Tumors and Acute Myeloid Leukemia
Read more: Hoth Therapeutics
- AN2 Therapeutics begins P-III part of a P-I/II study for Epetraborole to treat Treatment-Refractory MAC Lung Disease
Read more: AN2 Therapeutics
- AbbVie highlighted P-III (SEQUENCE) head-to-head study results of Skyrizi for Crohn's Disease, showed that risankizumab met both 1EPs of non-inferiority for clinical remission & superiority of endoscopic remission
Read more: AbbVie
- Neurocrine Biosciences highlighted P-III study (CAHtalyst) results of Crinecerfont for Congenital Adrenal Hyperplasia in adults which met its 1EPs at 24wk. & showed a significant percent reduction in daily glucocorticoid (GC) dose
Read more: Neurocrine Biosciences
Novartis highlighted P-III trial (NATALEE) results of Kisqali (ribociclib) for early breast cancer showed that patients maintained physical & social functioning for ~3yrs, presented at ESMO Virtual Plenary
Read more: Novartis

- The NICE has recommended Eli Lilly’s Tirzepatide for the treatment of adults with Type 2 Diabetes and Tirzepatide substantially declined blood sugar levels & body weight
Read more: Eli Lilly
- The US FDA has approved BioLineRx’s Aphexda (motixafortide) + Filgrastim for Multiple Myeloma, based on the 2-part P-III study (GENSIS) results
Read more: BioLineRx
- The US FDA has accepted the NDA for Verona Pharma’s Ensifentrine to treat Chronic Obstructive Pulmonary Disease, based on the P-III trials (ENHANCE-1 & 2)
Read more: Verona Pharma
- The US FDA has granted the Fast Track Designation to Affimed’s AFM13 + AlloNK for Relapsed or Refractory Hodgkin Lymphoma
Read more: Affimed
- The NICE has recommended Pfizer’s Vydura (rimegepant) for acute treatment of migraine
Read more: Pfizer
- The EMA’s CHMP has adopted a positive opinion recommending the approval of Ascendis Pharma’s TransCon PTH (palopegteriparatide) for Chronic Hypoparathyroidism, based on the P-III (PaTHway) & P-II (PaTH Forward) trials
Read more: Ascendis Pharma
- The NMPA has approved an IND Application of InnoCare’s ICP-189 + Furmonertinib to Initiate P-I study of ICP-189 + Furmonertinib for NSCLC Patients
Read more: InnoCare
- The EMA has adopted the positive opinion recommending approval of argenx’s Efgartigimod for Generalized Myasthenia Gravis, based on the P-III study (ADAPT-SC)
Read more: argenx

- Sandoz & Samsung Bioepis collaborated to commercialize SB17, a proposed biosimilar to Stelara in the US, Canada, EEA, Switzerland, and UK
Read more: Sandoz & Samsung Bioepis
- BioFactura highlighted P-I study results of BFI-751 (biosimilar, ustekinumab) that met the standard bioequivalence criteria
Read more: BioFactura

- Bausch + Lomb launches Miebo (perfluorohexyloctane ophthalmic solution) for Dry Eye Disease
Read more: Bausch + Lomb
- First Wave BioPharma Licenses Capeserod from Sanofi for Gastrointestinal Disorders
Read more: First Wave BioPharma and Sanofi
- AbbVie and Harpoon Therapeutics terminates its development and option agreement for HPN217
Read more: AbbVie and Harpoon Therapeutics
- Pharmazz and Sun Pharma collaborated to commercialize Sovateltide under the brand name of Tyvalzi in India
Read more: Pharmazz and Sun Pharma
- LIB Therapeutics & Hasten Biopharmaceutical collaborated to develop and commercialize Lerodalcibep in Greater China (Chinese Mainland, Hong Kong, Macau & Taiwan)
Read more: LIB Therapeutics & Hasten Biopharmaceutical
- Menarini & Astellas collaborated to commercialize Smyraf in Taiwan and selected South-East Asian markets. Menarini also gets an option to extend the rights to select South-East Asian markets
Read more: Menarini & Astellas

- Moderna collaborated with Immatics to develop innovative oncology therapies where Moderna will be responsible for the clinical development & commercialization of cancer vaccines and TCER therapeutics
Read more: Moderna and Immatics
- Cure Genetics & Frametact collaborated to develop gene therapy for Familial Neurological Diseases
Read more: Cure Genetics & Frametact

- Health Canada has authorized Moderna’s updated COVID-19 vaccines SPIKEVAX targeting the Omicron XBB.1.5 subvariant in individuals aged ≥6mos.
Read more: Moderna

- Zomedica commercialize its Truforma Diagnostic platform for diagnosis and management of Cushing’s Disease in Horses
Read more: Zomedica
Read more: PharmaShots Weekly Snapshots (September 04–08, 2023)














