PharmaShots Weekly Snapshots (Aug 11, 2025 – Aug 14, 2025)
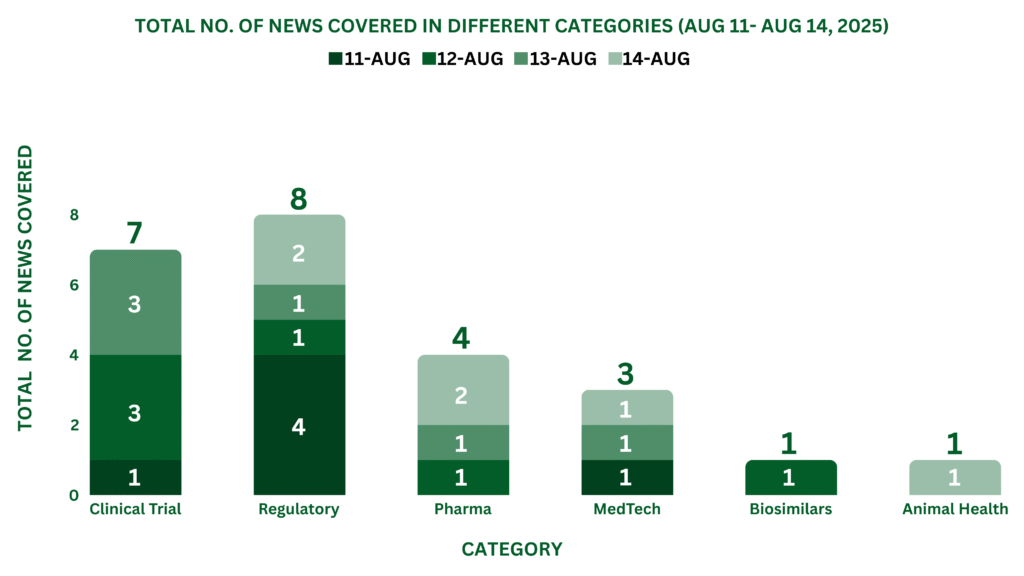
This week, PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, Animal Health, and Biosimilars. Check out our full report below:

Novartis Reports Topline P-III (NEPTUNUS-1 & 2) Trials Finding on Ianalumab to Treat Active Sjögren’s Disease
Read More: Novartis
Stoke Therapeutics and Biogen Report First Patient Dosing in P-III (EMPEROR) Trial of Zorevunersen for Dravet Syndrome
Read more: Stoke Therapeutics and Biogen
Novartis Reports Topline P-III (VAYHIT2) Trial Finding on Ianalumab Regimen to Treat Primary Immune Thrombocytopenia (ITP)
Read More: Novartis
Merck and Pfizer Report Topline P-III (KEYNOTE-905) Trial Finding on Keytruda + Padcev to Treat Muscle-Invasive Bladder Cancer (MIBC)
Read More: Merck and Pfizer
Greywolf Therapeutics Reports First Patient Dosing in P-I/II (EAST-1) Trial of GRWD0715 for Axial Spondyloarthritis
Read More: Greywolf Therapeutics
Remegen Reports P-III Trial Data of Telitacicept for Sjögren’s Syndrome in China
Read More: Remegen
Mabwell Reports First Patient Dosing in P-I Trial of Bulumtatug Fuvedotin for Triple-Negative Breast Cancer (TNBC) in the US
Read More: Mabwell

The US FDA Grants Accelerated Approval to Boehringer Ingelheim’s Hernexeos for HER2-Mutant NSCLC
Read More: BI
GSK Reports the US FDA’s sNDA Acceptance and Priority Review of Blujepa for Uncomplicated Urogenital Gonorrhoea
Read More: GSK
CSL Receives Health Canada’s Approval for Andembry as a Prophylactic Treatment of Hereditary Angioedema (HAE)
Read More: CSL
Arvinas Reports the US FDA’s NDA Acceptance of Vepdegestrant for ESR1-Mutated Breast Cancer
Read More: Arvinas
Junshi Biosciences Reports the NMPA’s sNDA Acceptance of Toripalimab + Disitamab Vedotin for Urothelial Carcinoma
Read More: Junshi Biosciences
Insmed Reports the US FDA’s Approval of Brinsupri (Brensocatib) for Non-Cystic Fibrosis Bronchiectasis (NCFB)
Read More: Insmed
Merck Receives Health Canada’s Approval for Perioperative Keytruda for Locally Advanced Head and Neck Squamous Cell Carcinoma (LA-HNSCC)
Read More: Merck
Sanofi’s Rilzabrutinib Secures the EMA’s Orphan Drug Designation to Treat IgG4-Related Disease
Read More: Sanofi

Expedition Therapeutics Enters a ~$645M Licensing Deal with Fosun Pharma for XH-S004
Read More: Expedition Therapeutics and Fosun Pharma
Bayer Enters a ~$1.3B Deal with Kumquat Biosciences to Develop and Commercialize a KRAS G12D Inhibitor
Read More: Bayer and Kumquat Biosciences
Basilea Pharmaceutica Enters Into a Licensing Deal with Venatorx Pharmaceuticals
Read More: Basilea Pharmaceutica and Venatorx Pharmaceuticals
Pilatus Biosciences Collaborates with Roche to Evaluate PLT012 & Tecentriq Combination for Hepatocellular Carcinoma (HCC)
Read More: Pilatus Biosciences and Roche

Nyxoah Receives the US FDA Approval for Genio System to Treat Obstructive Sleep Apnea
Read More: Nyxoah
Myra Vision Receives FDA Conditional IDE Approval for Calibreye TGT System to Treat Glaucoma
Read More: Myra Vision
BioCardia Collaborates with CART-Tech to Advance Heart3D Fusion Imaging for Interventional Cardiology
Read More: BioCardia and CART-Tech

Lupin Collaborates with Sandoz to Commercialize Ranibizumab Biosimilar
Read More: Lupin and Sandoz

PetPace Launches Epilepsy Insights Module for Canine Seizure Monitoring
Read More: PetPace
Related Post: PharmaShots Weekly Snapshots (Aug 04, 2025 – Aug 08, 2025)

