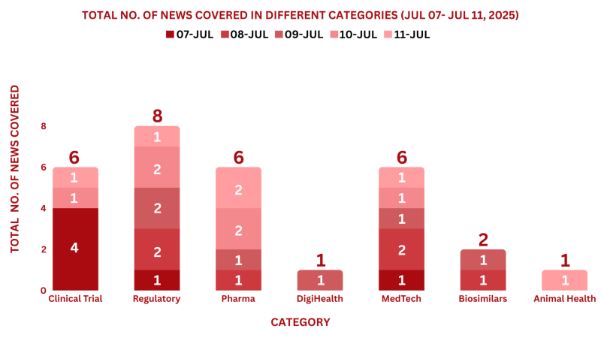
PharmaShots Weekly Snapshots (Jul 07, 2025 – Jul 11, 2025)
This week, PharmaShots’ news was all about the updates on clinical trials, Regulatory, Pharma, MedTech, M&A, and Biosimilars. Check out our full report below:

PulseSight Therapeutics Reports First Patient Dosing with PST-611 in P-I (PST-611-CT1) Trial for Dry AMD/Geographic Atrophy
Read More: PulseSight Therapeutics
BioAtla Presents Preliminary P-I Trial Data of BA3182 in Patients with Treatment Refractory Metastatic Adenocarcinoma at ESMO 2025
Read More: BioAtla
Novartis Reports Topline P-III (GCAptAIN) Trial Findings of Cosentyx (Secukinumab) for Giant Cell Arteritis
Read More: Novartis
Apogee Therapeutics Reports P-II (APEX) Trial Data on APG777 for Atopic Dermatitis (AD)
Read More: Apogee Therapeutics
LEO Pharma Reports Interim P-IIIb (ADHAND) Trial Data on Adbry for Atopic Dermatitis
Read More: LEO Pharma
Moleculin Receives RAMPA’s CTA Approval for P-IIb/III (MIRACLE) Study of AnnAraC in R/R AML
Read More: Moleculin

KalVista Reports the US FDA’s Approval of Ekterly (Sebetralstat) for Treating Hereditary Angioedema
Read More: KalVista
ImmunityBio’s Anktiva + BCG Receives the MHRA’s Approval for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer Carcinoma In Situ
Read More: ImmunityBio
Denali Therapeutics Reports the US FDA’s BLA Acceptance and Priority Review of Tividenofusp Alfa for Hunter Syndrome
Read More: Denali Therapeutics
J&J Reports US FDA’s sNDA Submission of Caplyta for Schizophrenia Relapse Prevention
Read More: J&J
Novo Nordisk Reports the EMA’s Application Submission for Higher Wegovy Dose for Weight Management
Read More: Novo Nordisk
Taiho Oncology (Part of Taiho Pharmaceutical) Reports the US FDA’s sNDA Acceptance of Inqovi + Venetoclax for Newly Diagnosed AML
Read More: Taiho Oncology
BeOne Medicines’ Tevimbra Receives the EC Approval for Nasopharyngeal Carcinoma
Read More: BeOne Medicines
Merck NDA for Investigational HIV-1 Two-Drug Regimen DOR/ISL Accepted by US FDA
Read More: Merck

Chugai Pharmaceutical Partners with Gero to Develop Age-Related Disease Therapies
Read More: Chugai Pharmaceutical and Gero
Alexion Partners with JCR Pharmaceuticals to Develop Genomic Medicines
Read More: Alexion and JCR Pharmaceuticals
Biocytogen Pharmaceuticals Enters a Licensing Agreement with BeOne Medicines for Multiple Antibody Candidates
Read More: Biocytogen Pharmaceuticals and BeOne Medicines
AbbVie Enters a Licensing Deal with IGI Therapeutics for ISB 2001
Read More: AbbVie and IGI Therapeutics
Eolas Therapeutics Joins Forces with AstraZeneca to Develop AZD4041
Read More: Eolas Therapeutics and AstraZeneca

Seegene Introduces STAgora Platform for Real-Time Infectious Disease Analysis
Read More: Seegene
Boston Scientific’s Farapulse PFA System Receives the US FDA Approval for Pulmonary Vein and Posterior Wall Ablation in Persistent AF Patients
Read More: Boston Scientific
Miach Orthopaedics Completes Patient Enrollment in BEAR MOON study of BEAR Implant for ACL Reconstruction
Read More: Miach Orthopaedics
Mendaera’s Focalist Robotic System Secures the US FDA 510(k) Clearance for Ultrasound-Guided Procedures
Read More: Mendaera
Exactech Reports the US FDA 510(k) Clearance of Equinoxe Scapula Reconstruction System for Acromial Stress Fractures
Read More: Exactech
Dymicron Secures US FDA IDE Approval for Triadyme-C Cervical Disc Study
Read More: Dymicron

Merck to Acquire Verona Pharma for ~$10B
Read More: Merck and Verona Pharma

Celltrion Launches Osenvelt and Stoboclo (Biosimilars, Xgeva and Prolia) in the US
Read More: Celltrion
Lupin Joins Forces with Zentiva to Develop TNF-Alpha Inhibitor Biosimilar
Read More: Lupin and Zentiva

FDA Approves Merck Animal Health’s Bravecto Quantum to Treat and Protect Dogs from Fleas and Ticks
Read More: Merck Animal Health

HealthBook+ Launches PaiGE for Real-Time Personalized Health Insights
Read More: HealthBook+
Related Post: PharmaShots Weekly Snapshots (Jun 30, 2025 – Jul 04, 2025)

