PharmaShots Weekly Snapshots (Jun 09, 2025 – Jun 13, 2025)
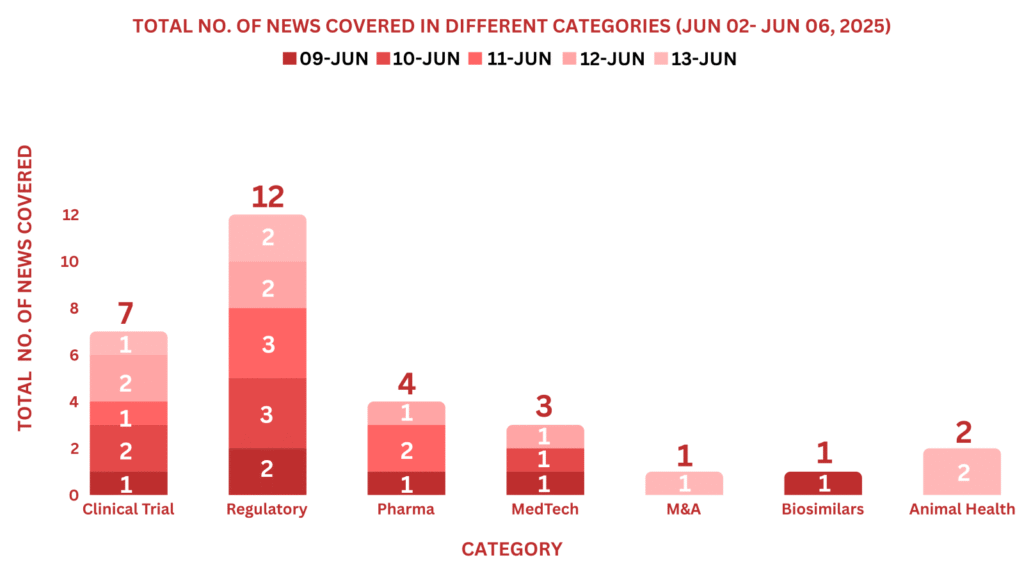
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Biosimilar & Animal Health. Check out our full report below:

Apellis Pharmaceuticals and Sobi Report P-III (VALIANT) Trial Data of Empaveli for C3 Glomerulopathy (C3G) and Primary IC-MPGN
Read More: Apellis Pharmaceuticals and Sobi
Daiichi Sankyo Reports First Patient Dosing in P-III (DESTINY-Endometrial01) Study Evaluating ENHERTU
Read More: Daiichi Sankyo
Regeneron Highlights Dupixent (dupilumab) Data From P-IV (DISCOVER) Study at RAD’25
Read More: Regeneron
ImmVira Reports First Patient Dosing in P-II Study Evaluating MVR-T3011 in Non-Muscle-Invasive Bladder Cancer (NMIBC) Patients
Read More: ImmVira
Johnson & Johnson Presents P-IIIb (APEX) Trial Findings of Tremfya for Active Psoriatic Arthritis at EULAR 2025
Read More: J&J
Novartis to Highlight P-IIIb Trial Data of Fabhalta for Paroxysmal Nocturnal Hemoglobinuria (PNH) at EHA 2025
Read More: Novartis
Celldex Highlights P-II Trial Data of Barzolvolimab for Chronic Spontaneous Urticaria at EAACI Congress 2025
Read More: Celldex

Arvinas and Pfizer Report the US FDA’s NDA Submission of Vepdegestrant for ESR1-Mutated Breast Cancer
Read More: Arvinas and Pfizer
Italfarmaco Obtains the EC’s Conditional Approval for Duvyzat to Treat Duchenne Muscular Dystrophy (DMD)
Read More: Italfarmaco
NMPA Accepts Marketing Authorization for Merck KGaA’s Pimicotinib for Tenosynovial Giant Cell Tumor (TGCT)
Read More: Merck KGaA
Alnylam Pharmaceuticals’ Amvuttra Receives the EC’s Approval for ATTR Amyloidosis with Cardiomyopathy (ATTR-CM)
Read More: Alnylam Pharmaceuticals
The US FDA Approves Merck’s ENFLONSIA for RSV Prevention in Infants
Read More: Merck
The US FDA Approves George Medicines’ Widaplik for Hypertension in Adults
Read More: George Medicines
Jazz Pharmaceuticals Reports the US FDA’s sNDA Acceptance and Priority Review of Zepzelca + Atezolizumab for ES-SCLC
Read More: Jazz Pharmaceuticals
Nuevocor Reports the US FDA’s IND Clearance of NVC-001 for LMNA DCM
Read More: Nuevocor
Nuvation Bio Reports the US FDA’s Approval of Ibtrozi to Treat Advanced ROS1+ NSCLC
Read More: Nuvation Bio
AbbVie’s Mavyret Receives the US FDA’s Approval for Acute Hepatitis C Virus
Read More: AbbVie
Moderna’s mRESVIA Receives the US FDA’s Approval for Respiratory Syncytial Virus (RSV) Disease
Read More: Moderna
UroGen Pharma Receives the US FDA’s Approval for Zusduri to Treat LG-IR-NMIBC
Read More: UroGen Pharma

Aytu BioPharma Collaborates with Fabre-Kramer Pharmaceuticals to Commercialize Exxua (Gepirone) in the US for Major Depressive Disorder
Read More: Aytu BioPharma and Fabre-Kramer Pharmaceuticals
OS Therapies & EVERSANA Partner to Commercialize OST-HER2 in the US for Pediatric Osteosarcoma
Read More: OS Therapies & EVERSANA
Philochem and RayzeBio Enter a Development and Commercialization Deal of ~$1.35B
Read More: Philochem and RayzeBio
Deep Apple Therapeutics Enters a Research Collaboration and Licensing Deal with Novo Nordisk to Advance Oral Small Molecules
Read More: Deep Apple Therapeutics and Novo Nordisk

LEX Diagnostics Seeks 510(k) Clearance and CLIA Waiver Status for its VELO system to Deliver Highly Sensitive PCR Results
Read More: LEX Diagnostics
TYBR Health’s B3 GEL System Receives the US FDA’s 510(k) Clearance to Protect Healing Tissue and Preserve Function
Read More: TYBR Health
Neurent Medical Receives the US FDA’s 510(k) Clearance of NEUROMARK System for Chronic Rhinitis
Read More: Neurent Medical

BioNTech to Acquire CureVac for ~$1.25B
Read More: BioNTech and CureVac

Samsung Bioepis Collaborates with NIPRO to Commercialize Biosimilar Candidates in Japan
Read More: Samsung Bioepis and NIPRO

The CVMP Adopts Positive Opinion on Elanco’s Zenrelia for Allergic and Atopic Dermatitis in Dogs
Read More: Elanco
Merck Animal Health Reports the CVMP Positive Opinion for Nobivac L6 and Nobivac LoVo L6 Vaccines for Canine Leptospirosis
Read More: Merck Animal Health
Related Post: PharmaShots Weekly Snapshots (Jun 02, 2025 – Jun 06, 2025)
