Insights+: The US FDA New Drug Approvals in June 2022
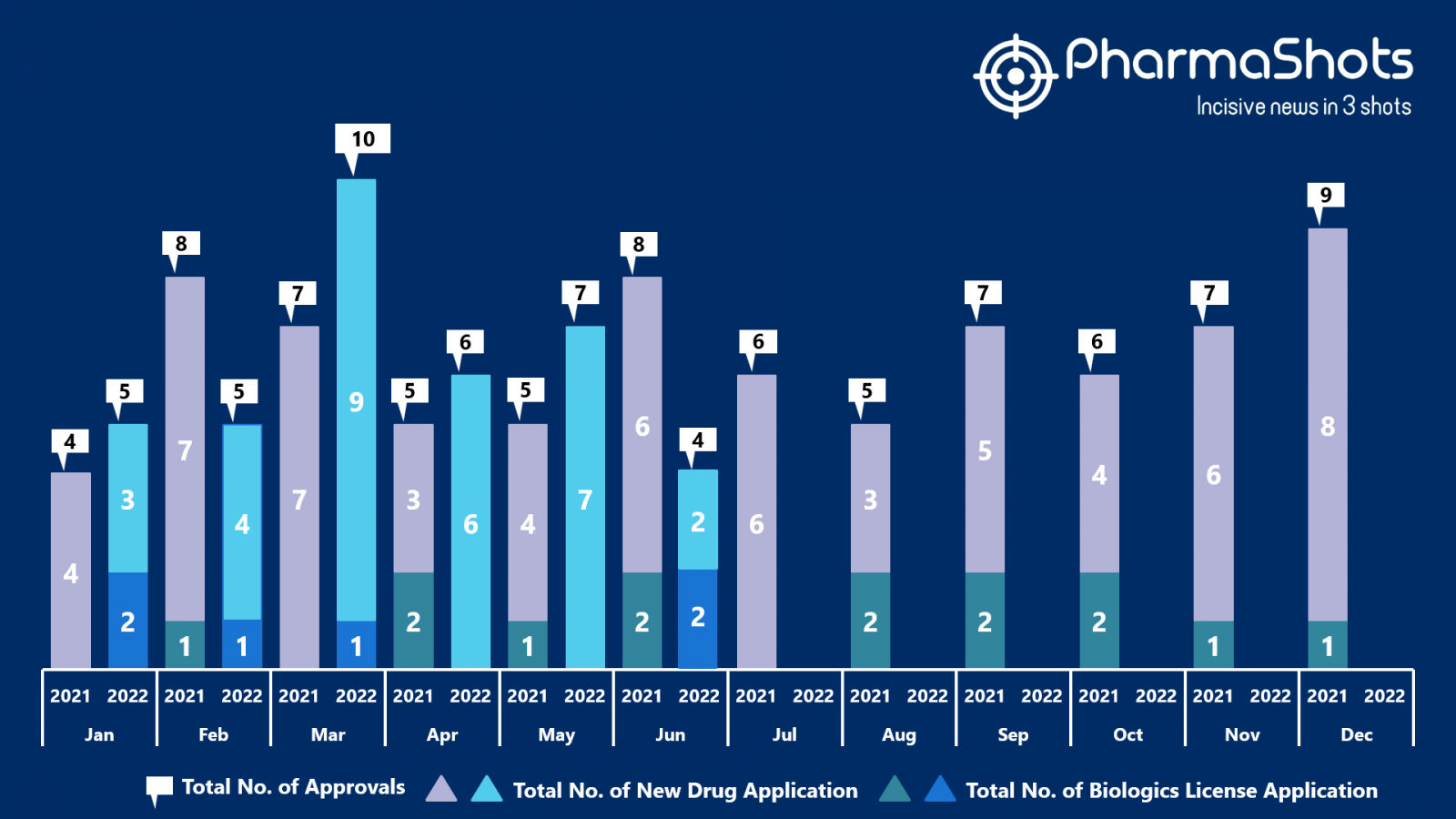
- The US FDA approved 2 NDAs and 2 BLA in June 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 37 novel products in 2022
- In June 2022, the major highlights drugs were Amvuttra’s approval for polyneuropathy of hereditary transthyretin-mediated amyloidosis, Skyrizi for active Crohn’s disease, Olumiant for severe alopecia areata
- PharmaShots has compiled a list of a total of 4 new drugs approved by the US FDA in June 2022
Amvuttra
Active ingredient: vutrisiran Approved: June 14, 2022
Company: Alnylam Pharmaceuticals Disease: Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis
- The approval was based on the 9mos. results from the P-III (HELIOS-A) study evaluate Amvuttra vs patisiran in a ratio (3:1) in 164 patients with hATTR amyloidosis with polyneuropathy. The product is expected to be available in early July
- The trial met the 1EPs & 2EPs i.e., improvement in mNIS+7 from baseline @9mos. (2.2point vs 14.8 point), 50% experienced an improvement in neuropathy impairment relative to baseline, Norfolk QoL-DN score & 10-MWT
- Improvements in exploratory EPs, and efficacy results were consistent with 9mos. data with no drug-related discontinuations or deaths. The company provides Alnylam Assist, a patient support program that helps patients access Amvuttra, verifies insurance benefits & offers financial assistance to qualified patients
Olumiant
Active ingredient: baricitinib Approved: June 14, 2022
Company: Eli Lilly and Incyte Disease: Severe Alopecia Areata
- The approval was based on the P-III (BRAVE-AA1 & 2) trials evaluating Olumiant (2/4mg, qd) vs PBO in 1200 adult patients with sev. AA
- The results from both studies showed that 17-22% of patients treated with Olumiant (2mg/day) & 32-35% with 4mg/day achieved ≥80% scalp hair coverage over 3-5% in PBO @36wks.; 11-13% & 24-26% vs 1-4% achieved ≥90% or hair coverage. Under the multiplicity control plan for BRAVE-AA2, the results for Olumiant 2mg/day were not statistically significant
- Improvements in eyebrow & eyelash coverage in patients with 4mg, qd dose @36wks., few patients discontinued treatment due to AEs. Lilly offers Olumiant Together support program to help patients access Olumiant treatment & provides a savings card for eligible commercially insured patients
Skyrizi
Active ingredient: risankizumab-rzaa Approved: June 20, 2022
Company: AbbVie Disease: Active Crohn’s Disease
- The approval was based on the P-III (ADVANCE) & (MOTIVATE) as induction studies & (FORTIFY) as maintenance therapy evaluating Skyrizi vs PBO in patients with active CD
- In the 12wk. induction studies, patients achieved endoscopic response & clinical remission while clinical response & clinical remission as early as 4wk. In the 52wk. maintenance trial, patients achieved the co-1EPs of endoscopic response & clinical remission after 1yr. while few or no symptoms were reported along with a reduction of visible signs of intestinal inflammation in both trials
- The company offers a patient support program & co-pay card to provide patients access to Skyrizi & other therapy, and reduce out-of-pocket costs for eligible, commercially-insured patients to $5/mos.
Breyanzi
Active ingredient: lisocabtagene maraleucel Approved: June 27, 2022
Company: BMS Disease: Large B-cell Lymphoma
- The approval was based on the P-III (TRANSFORM) study evaluating Breyanzi vs standard therapy regimens in adults with LBCL that was primary refractory or relapsed within 12mos. after 1L therapy
- The results showed improvements in EFS, CR & PFS; m-EFS (10.1mos. vs 2.3mos.); CR (66% vs 39%) with a median duration of CR (not reached); m-PFS (14.8mos. vs 5.7mos.), 97% received treatment over 47% who completed high-dose CT & autologous HSCT, well-established safety profile
- The approval was also based on the P-II (PILOT) study which showed deep & durable responses with ORR (80%), CR rate (54%) with a median time to CR of 1mos., m-DoR (11.2mos.) while m-DoR was not reached in those patients who achieved a CR
Related Post: Insights+: The US FDA New Drug Approvals in May 2022


