Insights+: The US FDA New Drug Approvals in May 2022
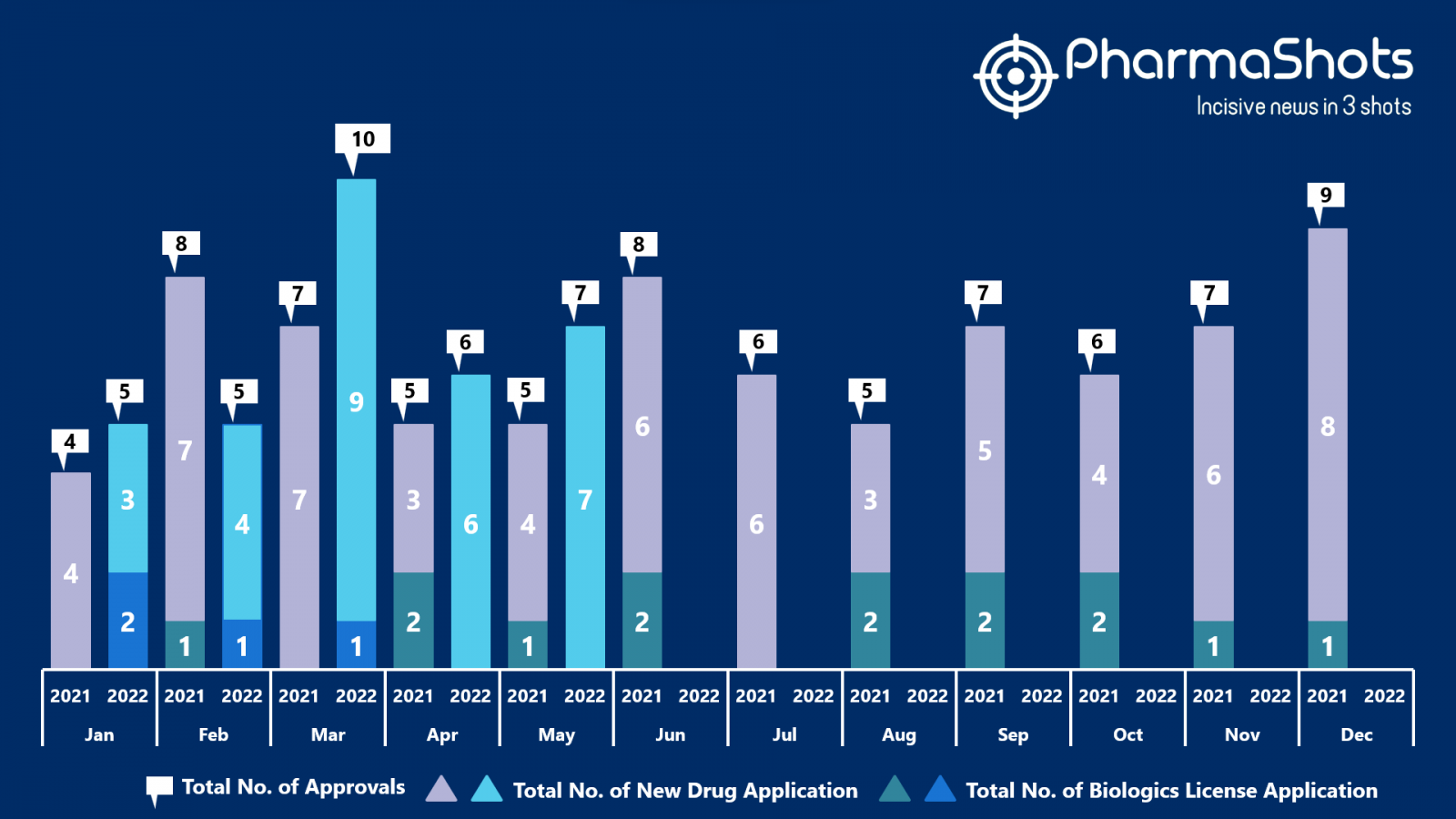
- The US FDA has approved 7 NDAs in May 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 33 novel products in 2022
- In May 2022, the major highlights drugs were Lilly’s Mounjaro (tirzepatide) received the US FDA’s approval for the treatment of adults with Type 2 Diabetes, Dermavant’s Vtama (tapinarof) Received the US FDA’s approval for the treatment of Plaque Psoriasis in adults
- PharmaShots has compiled a list of a total of 7 new drugs approved by the US FDA in May 2022
Voquezna Triple PAK & Voquezna Dual PAK
Active ingredient: vonoprazan; amoxicillin; clarithromycin Approved: May 03, 2022
Company: Phathom Pharmaceuticals Disease: H. pylori Infection
- The approvals were based on P-III (PHALCON-HP) trial evaluating Voquezna Triple & Dual PAK vs lansoprazole triple therapy in 1046 patients with H. pylori inf. The therapies are expected to be available in the US in Q3’22
- The results from both Voquezna treatment regimens showed non-inferiority over lansoprazole triple therapy in patients without a CAM or AMX resistant strain of H. pylori at baseline in modified ITT population, eradication rate (84.7% & 78.5% vs 78.8% & 78.8%), superior eradication rates over PPI-based triple therapy in patients with CAM resistant strains of H. pylori
- In the overall study population, eradication rate (80.8% & 77.2% vs 68.5% & 68.5%) who had a CAM-resistant strain of H. pylori (65.8% & 69.6% vs 31.9%, & 31.9%), respectively
Zydus’ Bortezomib Receives the US FDA’s Approval for the Treatment of Cancer
Velcade
Active ingredient: Bortezomib Approved: May 03, 2022
Company: Zydus Lifesciences Disease: H. pylori Infection
- The company has received the US FDA approval to manufacture and market Bortezomib for inj. 3.5 mg/vial (single-dose vial) to be bioequivalent and therapeutically equivalent to the Velcade to treat cancer incl. multiple myeloma and mantle cell lymphoma
- The drug will be manufactured at Zydus Hospira. The medication works by slowing or stopping cancer cell growth
- The company has manufacturing and research facilities in five Indian states: Gujarat, Maharashtra, Goa, Himachal Pradesh, and Sikkim along with US and Brazil. Zydus’ global business is well-established in regulated markets i.e., the US, EU (France and Spain), and the high-profile markets of Latin America and South Africa
Radicava ORS
Active ingredient: edaravone Approved: May 13, 2022
Company: Mitsubishi Tanabe Pharma America Disease: Amyotrophic Lateral Sclerosis
- The approval was based on the multiple studies i.e., 7 P-I pharmacology studies to evaluate PK, safety, drug-to-drug interactions, dosing, bioavailability & bioequivalence of Radicava ORS vs PBO along with P-III (MCI186-19) trial in patients with ALS. The therapy is as effective as the US FDA-approved Radicava
- The results showed a 33% reduction in loss of physical function @24wks. as measured by the ALSFRS-R. The P-III (MCI186-19) trial showed a safety & tolerability profile in 185 patients with ALS, AEs were reported in ≥10% of patients with Radicava
- The company launched the JourneyMate Support Program which provides educational support & resources to patients who are considering or have been prescribed an MTPA product
Mounjaro
Active ingredient: tirzepatide Approved: May 16, 2022
Company: Lilly Disease: Type 2 Diabetes
- The approval was based on the P-III (SURPASS) program that consists of (SURPASS-1/2/3/4/5) evaluating Mounjaro (5/10/15mg) as monothx. & as an add-on therapy vs Semaglutide, insulin glargine & insulin degludec in patients with T2D. The therapy is expected to be available in the US in the coming wks.
- The results showed avg. A1C reductions b/w 1.8% & 2.1% for Mounjaro 5mg & b/w 1.7% & 2.4% for 10 & 15mg dose while greater weight reductions in a 2EPs in all studies
- Tirzepatide is under regulatory review in the EU, Japan & multiple other countries. The company will engage with insurers, health systems & providers to offer patients access to Mounjaro & plans to offer a Mounjaro savings card to qualified patients
Siga’s Tpoxx (tecovirimat) Receives the US FDA’s Approval for the Treatment of Small Pox
Tpoxx
Active ingredient: tecovirimat Approved: May 19, 2022
Company: SIGA Technologies Disease: Small Pox
- The US FDA has approved Tpoxx as an IV formulation for smallpox caused by the variola virus in adults & pediatric patients. The therapy provides an alternative option who are not able to swallow oral capsules of Tpoxx
- The approval was based on animal efficacy studies in nonhuman primates & rabbits infected with nonvariola orthopoxviruses that were adequate & well-controlled
- Tpoxx is a novel small-molecule drug that prevents the formation of secondary viral envelopes in the variola virus and other poxviruses, thereby inhibiting viral maturation. The therapy has been approved in the US, Canada & EU for smallpox & is also indicated to treat monkeypox, cowpox & complications from immunization with vaccinia in the EU
United Therapeutics’ Tyvaso DPI Receives the US FDA’s Approval for Treatment of PAH and PH-ILD
Tyvaso DPI
Active ingredient: Treprostinil Approved: May 24, 2022
Company: United Therapeutics Disease: PAH and PH-ILD
- The approval was based on the (BREEZE) open-label study evaluating Tyvaso DPI in 51 patients with PAH. Additionally, commercialization activities are currently underway with patient availability is expected in June 2022
- The results from the patients with PAH who were transitioned from Tyvaso inhalation sol. to Tyvaso DPI demonstrated safety and tolerance @3wk. treatment period, improvements in six-minute walk distance, device preference and satisfaction & PROs
- Tyvaso DPI marks the first US FDA-approved dry powder inhaler therapy & is indicated for use in PAH and PH-ILD and represents a new formulation and inhalation device for inhaled treprostinil
Vtama
Active ingredient: tapinarof Approved: May 24, 2022
Company: Dermavant Sciences Disease: Plaque Psoriasis
- The P-III (PSOARING 1 & 2) trial met all 1EPs & 2EPs i.e., Vtama showed an improvement in PGA 6 score of “clear” or “almost clear” with a minimum 2-grade improvement (36% & 40% vs 6% & 6%) @12wks., ≥75% improvement in PASI-75 from baseline
- 92% were enrolled in the P-III LTE study who completed (PSOARING 1 & 2) trial, 40% of LTE study patients achieved complete disease clearance, remitting effect with a median duration of ~4mos. while in off-therapy was 130 days who achieved a clear skin PGA score of 0 or 1
- The safety & tolerability was consistent with PSOARING 1/2/3 study. Patient satisfaction data from the P-III LTE study showed that 81.7% considered it more effective than prior topical treatments. The product is expected to be available in June 2022
Related post: Insights+: The US FDA New Drug Approvals in April 2022





