Insights+: The US FDA New Drug Approvals in April 2022
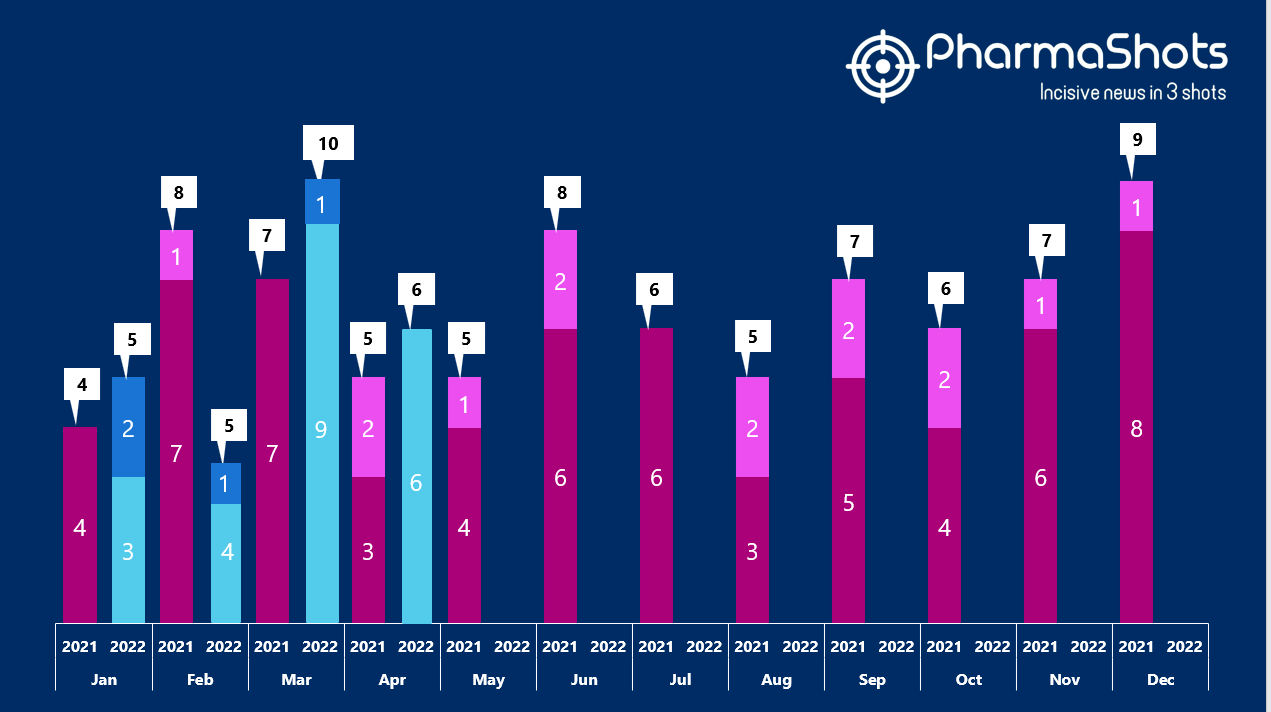
- The US FDA has approved 6 NDAs in Apr 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 26 novel products in 2022
- In Apr 2022, the major highlights drugs were Vivjoa for Recurrent Vulvovaginal Candidiasis, Cuvrior for Stable Wilson’s Disease, Igalmi for Acute Treatment of Agitation Associated with Schizophrenia or Bipolar I or II Disorder
- PharmaShots has compiled a list of a total of 6 new drugs approved by the US FDA in April 2022
Vijoice
Active ingredient: alpelisib Approved: April 7, 2022
Company: Novartis Disease: PIK3CA-Related Overgrowth Spectrum
- The US FDA approval was based on RWE from the (EPIK-P1) study evaluating Vijoice in adult & pediatric patients aged ≥2yrs. with PIK3CA-related overgrowth spectrum
- The results showed a 74% reduction in target lesion volume with a mean reduction of 13.7%, 27% with a confirmed response with ≥20% reduction in PROS target lesion volume @24wk., no patients experienced disease progression at the time of primary analysis
- Additionally, improvements in pain (90%), fatigue (76%), vascular malformation (79%), limb asymmetry (69%) & disseminated intravascular coagulation (55%). The company provides a patient support program including assistance to access medication, financial resources & continued education
Igalmi
Active ingredient: dexmedetomidine Approved: April 7, 2022
Company: BioXcel Therapeutics Disease: Agitation
- The approval was based on the (SERENITY I & II) studies evaluating Igalmi vs PBO in patients with agitation associated with schizophrenia & bipolar I or II disorder. The product is expected to be available in the US in Q2’22
- Both trials met its 1EPs & 2EPs i.e., both (120 & 180mcg) doses showed improvements from baseline along with a high response rate & demonstrated a rapid onset of action @20min. for both 180 & 120mcg doses in (SERENITY II) study while 20 & 30min. in (SERENITY I) study, respectively
- The results from the P-III (SERENITY II) trial in bipolar disorders were published in the JAMA. Igalmi can be self-administrated by patients under the supervision of a healthcare provider
Epsolay
Active ingredient: benzoyl peroxide Approved: April 25, 2022
Company: Sol-Gel Technologies and Galderma Disease: Inflammatory Lesions of Rosacea
- The approval was based on the two P-III trials to evaluate Epsolay vs vehicle cream in patients with inflammatory lesions of rosacea
- The coprimary EPs in both trials were the proportion of patients with treatment success & the absolute change from baseline in lesion counts @12wk. the therapy was effective @ 4wks. of treatment, 70% vs 38-46% reduction in inflammatory lesions of rosacea & 50% were clear or almost clear over 38-46% with PBO @12wk. Post-hoc analysis of lesion count & IGA success demonstrated a greater treatment effect as early as 2wk.
- In the OLE study, 73% were clear or almost clear @52wks. Epsolay (benzoyl peroxide, cream 5%) uses microencapsulation technology & is patent protected until 2040
Vivjoa
Active ingredient: oteseconazole Approved: April 28, 2022
Company: Mycovia Disease: Recurrent Vulvovaginal Candidiasis
- The approval was based on 3 P-III trials i.e., two (VIOLET) & 1 US (ultraviolet) study evaluating oteseconazole vs PBO in 875 patients with RVVC at 232 sites across 11 countries. Vivjoa is expected to be available in Q2’22
- The results from 2 (VIOLET) studies showed that (93.3% & 96.1% vs 57.2% & 60.6%) of women achieved a reduction of RVVC recurrence for 48wk. maintenance period. In the (ultraVIOLET) study, 89.7% vs 57.1% of women cleared initial yeast infection & did not have a recurrence for 50wk. maintenance period along with sustained efficacy
- Vivjoa is the 1st US FDA approved product & an azole antifungal that was indicated to reduce the incidence of RVVC in females
Camzyos
Active ingredient: mavacamten Approved: April 29, 2022
Company: BMS Disease: NYHA class II-III obstructive HCM
- The approval was based on the P-III (EXPLORER-HCM) trial to evaluate Camzyos vs PBO in a ratio (1:1) in 251 adult patients with NYHA class II-III obstructive HCM to improve functional capacity & symptoms
- The results showed that 37% vs 17% of patients achieved the composite 1EPs @30wk., greater improvement across all 2EPs including mean change from baseline post-exercise LVOT peak gradient & pVO2, improvement of NYHA class ≥1 (65% vs 31%)
- Mean change from baseline in KCCQ-23 † CSS, KCCQ-23 TSS & KCCQ-23 PL, mean resting LVEF was 74% at baseline in both groups, 6% vs 2% experienced reversible reductions in LVEF to <50%. The company offers multiple programs and resources along with patients support
Cuvrior
Active ingredient: mavacamten Approved: April 30, 2022
Company: Orphalan Disease: Stable Wilson’s Disease
- The US FDA has approved Cuvrior for the treatment of adult patients with stable WD who are de-coppered & tolerant to penicillamine
- The approval was based on data on the P-III (CHELATE) study to evaluate Cuvrior vs penicillamine in 53 adults with WD. The trial met its primary efficacy EPs i.e., Cuvrior was found to be non-inferior to penicillamine & showed that patients treated with Cuvrior had a similar mean NCC level to penicillamine @36wks., Cuvrior treated patients had a lower mean 24hr. urine copper excretion than penicillamine
- Cuvrior is a functionally scored tablet that contains 300mg trientine tetrahydrochloride. The product is expected to be available in early 2023
Related Post: Insights+: The US FDA New Drug Approvals in March 2022




