Insights+: EMA Marketing Authorization of New Drugs in February 2023
Shots:
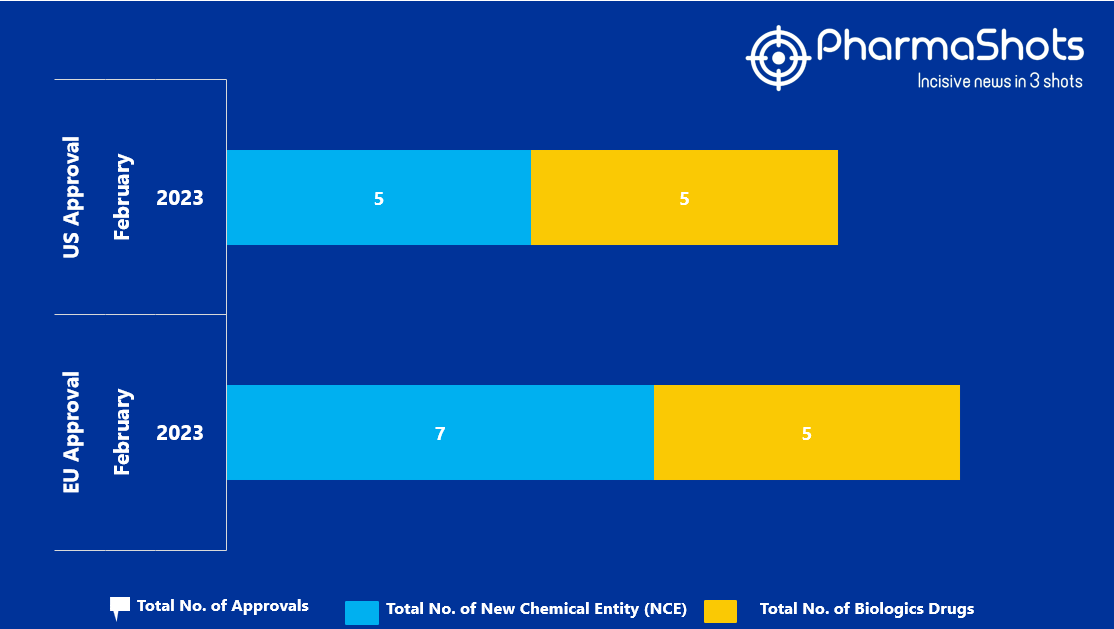
- The EMA approved 7 New Chemical Entity (NCE) and 5 Biologic Drugs in February 2023, leading to treatments for patients and advances in the healthcare industry
- In February 2023, the major highlights drugs were Forxiga’s Approval for symptomatic chronic heart failure, Fintepla for adjunctive treatment of seizures associated with lennox-gastaut syndrome
- PharmaShots has compiled a list of a total of 12 new drugs approved by the EMA in February 2023
Hemlibra
Active ingredient: emicizumab Approved: February 01, 2023
Company: Roche Disease: Haemophilia A
- The EC has approved a label expansion of Hemlibra (bispecific factor IXa- and factor X-directed Ab) in patients with a mod. haemophilia A. The approval was based on the results of the P-III trial (HAVEN 6) evaluating the safety, efficacy, PK & PD of Hemlibra
- The results showed effective bleed control & favorable safety profile, 70.8% had mod. haemophilia A without factor VIII inhibitors with no new safety signals, patients experienced no bleeds/no spontaneous bleeds/no joint bleeds were 66.7%/81.9%/88.9%, respectively
- The decision was also based on real-world data while the label expansion will provide an effective & convenient prophylactic treatment option for patients with a mod. haemophilia A with a sev. bleeding phenotype
Forxiga
Active ingredient: dapagliflozin Approved: February 07, 2023
Company: AstraZeneca Disease: Heart Failure
- The approval was based on the P-III trial (DELIVER) evaluating Forxiga vs PBO in 6263 HF patients with LVEF ≥40% with/out T2D. The primary composite EPs were the time to the first occurrence of CV death, hHF, or an urgent HF visit. & 2EPs incl. the total no. of HF events & CV death, change from baseline in the total symptom score of KCCQ @8mos., time to the occurrence of CV death & death from any cause
- Forxiga was found to be 1st HF drug to show a mortality benefit across the full ejection fraction range in the prespecified pooled analysis of the P-III (DELIVER) & (DAPA-HF) trials
- Forxiga was approved in 100+ countries globally for T2D, HFrEF & CKD incl. the US, the EU, China & Japan. The therapy has received regulatory approvals in Great Britain, Japan & Turkey
Hemgenix
Active ingredient: etranacogene dezaparvovec Approved: February 21, 2023
Company: CSL Disease: Hemophilia B
- The EC has granted CMA for Hemgenix in adults with sev. & moderately sev. hemophilia B without a history of Factor IX inhibitors
- The approval was based on the P-III trial (HOPE-B) results evaluating Hemgenix in 54 adult patients. The results showed a stable & durable increase in mean Factor IX activity levels with a mean Factor IX activity of 36.9%, leading to an ABR reduction of 64%, 96% discontinued routine Factor IX prophylaxis & 97% reduction in mean Factor IX consumption @18mos. post-treatment, was well-tolerated with no serious TRAEs
- The EC’s decision will be valid in all EU member states, incl. EEA countries of Iceland, Norway & Liechtenstein. Hemgenix’s submission is currently under MHRA review in the UK
Imfinzi
Active ingredient: durvalumab Approved: February 22, 2023
Company: AstraZeneca Disease: Liver and Non-Small Cell Lung Cancer
- The approval was based on the P-III trial (HIMALAYA) evaluating Imjudo (300mg) + Imfinzi (1500mg, q4w) vs sorafenib in 1324 patients with HCC & P-III (POSEIDON) trial of Imfinzi + Imjudo & CT vs CT alone in 1013 patients with metastatic NSCLC
- In (HIMALAYA) & (POSEIDON) trials published in the NEJM & Journal of Clinical Oncology, 22% & 23% reduction in risk of death, m-OS (16.4 vs 13.8mos.) & (14.0 vs 11.7mos.), patients were alive (31% vs 20%) @3yrs. & (33% vs 22%) @2yrs. & no new safety signals were seen
- In (POSEIDON) trial, 28% reduction in risk of disease progression or death, m-PFS (6.2 vs 4.8mos.) & updated results @4yrs. of follow-up showed a sustained survival benefit, 25% reduction in risk of death, m-OS (14 vs 11.7mos.), 25% vs 13.6% were alive @3yr.
Triumeq PD
Active ingredient: dolutegravir, abacavir and lamivudine Approved: February 22, 2023
Company: ViiV Healthcare Disease: Human Immunodeficiency Virus Type 1
- The company received marketing authorization from the EC for Triumeq PD to treat paediatric patients with HIV-1. The authorization also includes the label extension of Triumeq & lowers the minimum weight of a child who can be prescribed this medicine to 25kgs from 40 kg
- The authorization was based on the US FDA’s approval of Triumeq PD in 2022. In 2021, 52% of children aged ≤14yrs. living with HIV had access to antiretroviral medications with paediatric optimizations.
- Triumeq is a dispersible tablet formulation of the fixed-dose combination of abacavir, dolutegravir, and lamivudine for the treatment of paediatric patients with HIV-1
Akeega
Active ingredient: niraparib Approved: February 24, 2023
Company: Janssen Disease: Castration-Resistant Prostate Cancer
- The opinion was based on the P-III study evaluating niraparib (200mg, qd) + abiraterone acetate & prednisone vs PBO + AA & prednisone in a ratio (1:1) in 765 patients with/out certain HRR gene alterations
- Improvement in rPFS in all HRR+ patients & 47% risk reduction for rPFS in patients with BRCA1/2 gene mutations. In the updated 2nd interim analysis, consistent & significant treatment effect in rPFS at a median follow-up of 24.8mos. in the BRCA subgroup with an m-rPFS (19.5 vs 10.9mos.)
- In the BRCA subgroup, improved OS, time to symptomatic progression & consistent improvement of TCC were reported, grade 3/4 AEs for HRR gene alterations (67% vs 46.4%), and AEs leading to treatment discontinuation (10.8% vs 4.7%) & maintained overall QoL
Vafseo
Active ingredient: vadadustat Approved: February 24, 2023
Company: Akebia Disease: Chronic Kidney Disease
- The EMA’s CHMP has adopted a positive opinion recommending approval of Vafseo (HIF-PH inhibitor) for symptomatic anemia associated with CKD in adults on chronic maintenance dialysis. The EC’s decision is expected in ~2mos.
- The opinion was based on the comprehensive development program incl. the P-III (INNO2VATE) program of vadadustat. The trial met its primary & secondary efficacy EPs in each of 2 (INNO2VATE) studies i.e., (Correction/Conversion and Conversion) & showed non-inferiority to darbepoetin alfa
- The therapy also achieved primary safety EPs i.e., non-inferiority of vadadustat over darbepoetin alfa in time to 1st occurrence of major adverse cardiovascular EPs. The EC’s decision will valid for all 27 EU member states, Iceland, Norway & Liechtenstein
Opzelura
Active ingredient: ruxolitinib Approved: February 24, 2023
Company: Incyte Disease: Non- Segmental Vitiligo
- The EMA’s CHMP has issued a positive opinion recommending the approval of Opzelura for non-segmental vitiligo in adults & adolescents aged 12yrs. with facial involvement
- The opinion was based on the 2 P-III trials (TRuE-V1 & TRuE-V2) evaluating ruxolitinib cream vs vehicle in 600+ patients. The results showed a significant improvement in facial & total body repigmentation as shown by the no. of patients reaching the F-VASI-T-VASI EPs @24wk. with a higher proportion of patients responding at 52wk.
- Opzelura was approved in the US for the topical treatment of nonsegmental vitiligo & is also approved for the topical short-term & non-continuous chronic treatment of mild to mod. AD in non-immunocompromised patients
PRX–102
Active ingredient: pegunigalsidase alfa Approved: February 25, 2023
Company: Protalix BioTherapeutics Disease: Fabry Disease
- The EMA’s CHMP has adopted a positive opinion recommending marketing authorization for PRX-102 to treat adult patients with Fabry disease. The EC’s final decision on the MAA is expected in May 2023
- The opinion was based on positive data from a comprehensive set of preclinical, clinical & manufacturing studies & clinical programs incl. P-III (BALANCE), (BRIDGE) & (BRIGHT) trials, the P-I/II trial, and ongoing related extension studies. The tolerability & immunogenicity profiles of PRX-102, acc. to data from the clinical program suggested that it has the potential to be a long-lasting therapy
- PRX–102 is a novel recombinant human α–Gal–A enzyme that is being investigated as an enzyme replacement therapy (ERT) for Fabry disease
Libtayo
Active ingredient: cemiplimab Approved: February 27, 2023
Company: Regeneron Disease: Non-Small Cell Lung Cancer
- The EMA’s CHMP has adopted a positive opinion of Libtayo + Pt-based CT for advanced NSCLC with ≥1% PD-L1 expression. The EC’s final decision is expected in the coming months
- The opinion was based on the P-III trail (EMPOWER-Lung 3) evaluating Libtayo + Pt-doublet CT vs Pt-doublet CT alone in 466 patients with LA or metastatic NSCLC, sq. or non-sq. histologies across all PD-L1 expression levels with no ALK, EGFR, or ROS1 aberrations
- The trial showed an improvement in OS in the overall population, m-OS (22 vs 13mos.) with a median follow-up of 16mos., 45% relative reduction in risk of death, and survival benefit was observed with a median duration of follow-up of 28mos., AEs (25%) of patients and permanent discontinuation due to AEs in 5% of patients
11. AbbVie Receives EMA’s CHMP Positive Opinion of Rinvoq (upadacitinib) for Crohn’s Disease
Rinvoq
Active ingredient: upadacitinib Approved: February 27, 2023
Company: AbbVie Disease: Crohn’s Disease
- The EMA’s CHMP adopted the positive opinion recommending the approval of upadacitinib for moderately to severely active CD
- The opinion was based on 2 induction studies (U-EXCEED & U-EXCEL) & 1 maintenance study (U-ENDURE) evaluating upadacitinib (45mg, qd) as IT & 15/30mg, qd as MT vs PBO. In all P-III studies, patients achieved the co-primary EPs of clinical remission per SF/AP & endoscopic response
- Patients also achieved the 2EPs of endoscopic remission, SES-CD ulcerated surface subscore of 0 @12 & 52wks. in patients with SES-CD ulcerated surface subscore ≥1 at baseline, mucosal healing is connected to improvements seen by endoscopy & absence or disappearance of ulceration. The safety profile was consistent with the known safety profile of upadacitinib
Fintepla
Active ingredient: fenfluramine Approved: February 28, 2023
Company: UCB Disease: Seizures
- Fintepla has been approved in the EU for seizures associated with LGS as an add-on therapy to other anti-epileptic medicines in patients aged ≥2yrs.
- The approval was based on safety & efficacy data from a P-III clinical trial evaluating Fintepla vs PBO in 263 patients which showed a greater reduction in the frequency of drop seizures at a dose of 0.7mg/kg/day while no cases of valvular heart disease or pulmonary arterial hypertension were reported
- Fenfluramine is a serotonin-releasing agent that stimulates multiple 5-HT receptor sub-types through the release of serotonin. To ensure consistent cardiac monitoring & to reduce potential off-label use, fenfluramine oral solution is available through a controlled access program
Note: PRX–102, Akeega, Vafseo, Opzelura, Libtayo & Rinvoq received EMA’s CHMP Positive Opinion & EC’s Conditional Marketing Authorization for Hemgenix, Triumeq PD
Related Post: Insights+: EMA Marketing Authorization of New Drugs in January 2023





