New Drug Designations – December 2024
Shots:
- PharmaShots’ designation report provides a concise overview of the latest drug designations by major regulatory authorities, including the FDA, EMA, MHLW and NMPA
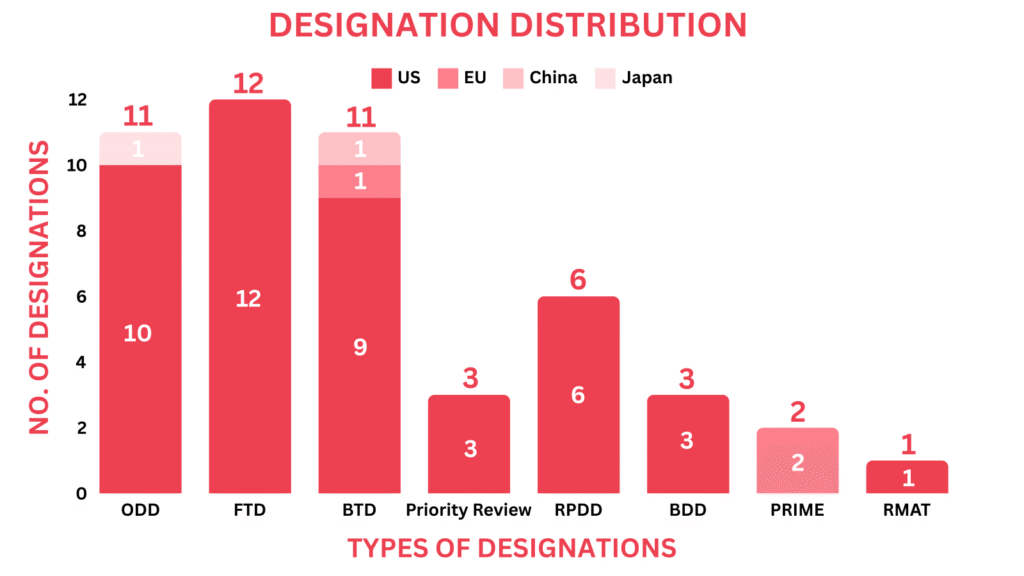
- The December 2024 report covers designations granted to 43 drugs and 3 devices, encompassing 13 small molecules, 10 biologics, 7 cell and gene therapies & 3 medical devices among others
- Significant trends this month show, Nobias Therapeutics’ NB-001 secured the US FDA’s ODD and RPDD to treat DiGeorge syndrome

Ersodetug – Biologic
| Sponsor | Rezolute |
| Indication | Hypoglycemia |
| Phase | P-III |
| MOA | Insulin Receptor Antagonists |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 03, 2024 |
- The US FDA has granted ODD to ersodetug for the treatment of hypoglycemia caused by tumor HI
- The company is expecting to initiate a P-III trial assessing ersodetug in tumor HI patients in 2025 with real-world data exhibited through an expanded access program
- Ersodetug is a fully human mAb that opposes the effects of over-activated insulin receptors by binding to its unique allosteric site
NB-001 – Antivirals
| Sponsor | Nobias Therapeutics |
| Indication | DiGeorge syndrome (22q11.2 Deletion Syndrome) |
| Phase | P-II |
| MOA | mGluR Modulator |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 05, 2024 |
- The US FDA has granted ODD & RPDD to NB-001 for the treatment of patients with neuropsychiatric symptoms related to 22q11.2 deletion syndrome (DiGeorge syndrome/22q11DS)
- NB-001 is currently under investigation in a P-II trial assessing NB-001 vs PBO in 22q11DS patients with top-line results presented at CNS Annual Meeting, 2023
- NB-001 (mGluR modulator) disrupts neuronal signaling that relieves neuropsychiatric symptoms
Crofelemer – Small molecule
| Sponsor | Jaguar Health |
| Indication | Diarrhea |
| Phase | P-II |
| MOA | Chloride channel antagonists |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 17, 2024 |
- The US FDA has granted ODD to crofelemer for treating diarrhea in cholera patients
- Crofelemer is under investigation in 3 IIT PoC & 2 P-II studies for SBS-IF ± MVID in the US, EU &/or Middle East/North Africa regions. The first dosing for the same is expected in Dec’24 & Q1’25, whereas IIT PoC results are expected in Q2’25
- Additionally, the company intends to pursue ODD & a Tropical Disease Priority Review Voucher for NP-300 to treat diarrhea in cholera patients
ST316 – Peptides
| Sponsor | Sapience Therapeutics |
| Indication | Familial Adenomatous Polyposis |
| Phase | P-II |
| MOA | β-catenin Antagonist |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 19,2024 |
- The US FDA has granted ODD to ST316 for the treatment of patients with familial adenomatous polyposis (FAP)
- ST316 is currently in the P-II study of Phase 1-2 (ST316-101) trial assessing ST316 with relevant SoC & multiple lines of treatment in CRC patients, while the P-I was the dose escalation study in patients having advanced solid tumors with Wnt/β-catenin signaling pathway abnormalities incl. CRC
- ST316 (β-catenin & BCL9 antagonist) selectively inhibits Wnt/β-catenin signaling pathway responsible for FAP & >80% of CRCs in patients
Umbilical Cord Outer Lining Stem Cells (UCSLs) – Cell Therapy
| Sponsor | Restem |
| Indication | Polymyositis & Dermatomyositis |
| Phase | P-I |
| MOA | Unknown |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 03, 2024 |
- The US FDA has granted ODD to UCSLs programs for the treatment of Polymyositis (PM) & Dermatomyositis (DM) patients
- The therapy demonstrated positive results in P-I study with significant clinical improvements; favorable safety & efficacy plus probable decrease in the need of steroidal therapies. The initiation of P-II/III trials is expected in Q1’2025
BRC-002 – Cannabinoid
| Sponsor | Biopharmaceutical Research Company |
| Indication | Complex Regional Pain Syndrome (CRPS) |
| Phase | P-I |
| MOA | Undefined |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 03, 2024 |
- The US FDA has granted ODD to BRC-002 for the treatment of complex regional pain syndrome (CRPS) that is currently under investigation in the IIT P-I study
- The company plans to initiate patient enrollment for the P-II study of BRC-002 by the YE’25 & seeks FDA input on the development plan
S
Azeliragon – Small Molecule
| Sponsor | Cantex Pharmaceuticals |
| Indication | Brain Metastasis |
| Phase | P-I |
| MOA | RAGE antagonist |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 09, 2024 |
- The US FDA has granted ODD to azeliragon for the treatment of patients with brain metastasis from breast cancer
- Azeliragon (oral, QD) inhibits RAGE interactions with its ligands (incl. HMGB1 & S100 proteins) & is under investigation for brain metastasis, glioblastoma, breast cancer, pancreatic cancer, & hospitalized pneumonia patients
AVG-002 – Gene Therapy
| Sponsor | AlveoGene |
| Indication | Neonatal SP-B Deficiency |
| Phase | Preclinical |
| MOA | Gene transference |
| RoA | Inhalation |
| Approval Authority | FDA |
| Date | Dec 03, 2024 |
- The US FDA has granted ODD to AVG-002 for neonatal SP-B deficiency, following the grant of RPDD in Nov’24. AVG-002 is anticipated to enter clinical development & filing is expected by 2028
- The Preclinical data showed increased survivability in SP-B deficient murine model with a single dose of AVG-002 plus recovery of lung histology & function in disease-induced tissue after AVG-002 treatment
- AVG-002, an inhalable gene therapy, is developed using AlveoGene’s InGenuiTy platform that utilizes a pseudo-typed lentiviral vector to efficiently deliver the gene directly to the alveolar region of the lung
Manufactured Red Blood Cells – Cell Therapy
| Sponsor | Safi Biotherapeutics |
| Indication | Sickle Cell Disease |
| Phase | Preclinical |
| MOA | Unknown |
| RoA | Unknown |
| Approval Authority | FDA |
| Date | Dec 18, 2024 |
- The US FDA has granted ODD & RPDD to manufactured RBCs (mRBCs) for use in chronic transfusion to treat sickle cell patients
- The company is advancing its validation of mRBCs cGMP manufacturing for clinical studies & anticipates initiating the trials by 2027, post-completion of IND-enabling activities
VGT-1849A – antisense oligonucleotide
| Sponsor | Vanda Pharmaceuticals |
| Indication | Polycythemia Vera |
| Phase | Preclinical |
| MOA | JAK2 inhibitor |
| RoA | Unknown |
| Approval Authority | FDA |
| Date | Dec 20, 2024 |
- The US FDA has granted ODD to VGT-1849A for the treatment of patients with polycythemia vera (PV)
- VGT-1849A is a selective ASO-based JAK2 inhibitor with the potential to decrease JAK2V617F-driven pathogenic signaling, thereby reducing malignant proliferation & survivability of hematopoietic cells
Sparsentan – Small Molecule
| Sponsor | Renalys & Travere |
| Indication | IgA nephropathy |
| Phase | P-III |
| MOA | Angiotensin II & Endothelin A receptor antagonists |
| RoA | Oral |
| Approval Authority | MHLW |
| Date | Dec 02, 2024 |
- The Japanese MHLW has granted ODD to sparsentan, being investigated under P-III study in Japan for treating IgA nephropathy. It was FDA approved under brand name Filspari for slowing kidney function among adults in Sep 2024
- Renalys secured its development & commercialization rights from Travere Therapeutics across Japan, South Korea, Taiwan, Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand & Vietnam as per an agreement signed in Jan 2024
- Filspari (QD, oral) is a non-immunosuppressive medication for IgAN that blocks endothelin-1 and angiotensin II pathways to reduce glomerular injury

PT217 – Biologic
| Sponsor | PTC Therapeutics |
| Indication | Neuroendocrine Prostate Cancer |
| Phase | P-II |
| MOA | CD47 & DLL3 Inhibitors |
| RoA | Parenteral |
| Approval Authority | FDA |
| Date | Dec 4, 2024 |
- The US FDA has granted FTD to PT217 for treating metastatic de novo or treatment-emergent neuroendocrine prostate cancer (NEPC)
- PT217 is being investigated under P-I/II (SKYBRIDGE) trial for its safety, tolerability, pharmacokinetics and preliminary efficacy in patients with advanced or refractory cancers with DLL3 expression and another P-I trial in China (CTR20242720)
- PT217 is a first-in-class bispecific antibody targeting DLL3 & CD47 and being developed for SCLC & neuroendocrine carcinoma (NEC), incl. NEPC. Phanes has also partnered with Roche to study it in combination with atezolizumab
S
AdAPT-001 – Immunotherapy
| Sponsor | EpicentRx |
| Indication | Soft Tissue Sarcoma |
| Phase | P-II |
| MOA | TGFβ inhibitors |
| RoA | Intratumoral |
| Approval Authority | FDA |
| Date | Dec 5, 2024 |
- The US FDA has granted FTD to AdAPT-001 + nivolumab/atezolizumab for the treatment of r/r advanced or metastatic soft tissue sarcoma (STS) post disease progression after at least 1L of therapy
- The Designation was supported by data from P-I/II trials assessing AdAPT-001 as monotherapy or in combination with PD-1/PD-L1 inhibitors showed PFS of ~8.5mos. in patients with STS & other tumors
- AdAPT-001 is an oncolytic adenovirus-delivered TGF-βR inhibitor that neutralizes TGFβ locally to decrease T cell function & sensitize STS tumors to checkpoint inhibitors
Fluzone & Flublok + Novavax – Vaccine
| Sponsor | Sanofi |
| Indication | Influenza and COVID-19 |
| Phase | P-I/II |
| MOA | Immunostimulants |
| RoA | Intramuscular |
| Approval Authority | FDA |
| Date | December 11, 2024 |
- The US FDA has granted FTDs to two combinations, Fluzone (influenza protein-based trivalent vaccine) + Novavax (COVID-19 vaccine) & Flublok + Novavax, for preventing influenza and COVID-19 infections in subjects (50+ yrs.)
- Fluzone High-Dose and Flublok outperformed standard-dose flu vaccines in preventing influenza and reducing flu-related hospitalizations in older adults. The Novavax COVID-19 vaccine has shown better tolerability as a booster and high efficacy as primary vaccination in P-III studies
- Sanofi has launched 2 P-I/II studies to assess the safety and immune response of combination vaccines to prevent influenza A, influenza B & COVID-19
- NCT06695117: Combines Fluzone High-Dose (TIV-HD) with the Novavax COVID-19 vaccine for individuals aged 50+ yrs.
- NCT06695130: Combines Flublok (RIV3) with the Novavax COVID-19 vaccine for the same age group
IMM-1-104 – Small Molecule
| Sponsor | Immuneering |
| Indication | Advanced Melanoma |
| Phase | P-IIa |
| MOA | MAPK Inhibitor |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 12, 2024 |
- The US FDA has granted FTD to IMM-1-104 for the treatment of unresectable or metastatic NRAS-mutant melanoma patients progressed or intolerable to PD-1/PD-L1 checkpoint inhibitors
- IMM-1-104 (QD) inhibits MAPK pathway to stimulate RAS activity that impacts cancer cells selectively & is in P-IIa trial for the treatment of advanced solid tumors incl. melanoma
LPCN 1148 – Small Molecule
| Sponsor | Lipocine |
| Indication | Sarcopenia |
| Phase | P-II |
| MOA | Androgen receptor agonists |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 17, 2024 |
- The US FDA has granted FTD to LPCN 1148 for the treatment of sarcopenia in decompensated cirrhosis patients
- LPCN 1148 was subjected to a recent PoC P-II study in decompensated cirrhosis patients, showing improved sarcopenia & related clinical outcomes
- LPCN 1148 (oral) contains testosterone dodecanoate (androgen receptor agonist) & utilizes multimodal approach to manage cirrhosis & related comorbidities
NX-5948 – Small Molecule
| Sponsor | Nurix Therapeutics |
| Indication | R/R Waldenstrom’s Macroglobulinemia |
| Phase | P-II |
| MOA | Bruton’s Tyrosine Kinase Degrader |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 19, 2024 |
- The US FDA has granted FTD to NX-5948 for the treatment of r/r Waldenstrom’s macroglobulinemia (WM) in patients who received at least 2L of treatment, incl. a BTK inhibitor
- The designation is followed by P-I data evaluating the safety & efficacy of NX-5948 & the company is enrolling WM patients in a P-Ib (expansion cohort) trial with results expected in 2025
- NX-5948 is a novel oral small molecule that degrades BTK proteins through cereblon E3 ligase complex without affecting other cereblon neo-substrates
BGC101 – Cell Therapy
| Sponsor | BioGenCell |
| Indication | Critical Limb Threatening Ischemia |
| Phase | P-II |
| MOA | Cell replacements |
| RoA | Intramuscular |
| Approval Authority | FDA |
| Date | Dec 19, 2024 |
- The US FDA has granted FTD to BGC101 for the treatment of Critical Limb Threatening Ischemia (CLTI) patients to prevent amputations, disease progression & relieve pain
- BGC101 is under investigation in the P-II trial, which completed patient recruitment & is being conducted in the US, EU & Israel
- BGC101 utilizes BioGenCell’s TRACT platform to stimulate tissue regeneration in the damaged limb by developing personalized cell therapies through the immune & stem cells obtained from the patient
SC291 – Cell Therapy
| Sponsor | Sana Biotechnology |
| Indication | B-cell mediated autoimmune disease |
| Phase | P-I |
| MOA | Immunologic cytotoxicity |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 2, 2024 |
- The US FDA has granted FTD to SC291 for the treatment of r/r SLE, incl. extrarenal lupus & lupus nephritis
- SC291 is being evaluated in the GLEAM trial in B-cell mediated autoimmune disease patients (incl. lupus nephritis, extrarenal lupus, plus ANCA-associated vasculitis with ongoing enrolment & initial results expected in 2025
- SC291, an allogeneic CAR T cell therapy, is developed using Sana’s hypoimmune platform that targets CD19 protein expressed on the surface of B cells
R289 – Small Molecules
| Sponsor | Rigel Pharmaceuticals |
| Indication | Lower-risk Myelodysplastic Syndrome |
| Phase | P-I |
| MOA | IRAK1/4 dual inhibitor |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 2, 2024 |
- The US FDA has granted FTD to R289 in patients with previously-treated transfusion dependent lower-risk myelodysplastic syndrome (LR-MDS)
- The P-Ib study is currently assessing the safety, tolerability, pharmacokinetics & preliminary efficacy of R289 among patients with r/r lower-risk MDS, with recruitment underway
- R289 (prodrug of R835) is an IRAK1/4 dual inhibitor that works by blocking inflammatory cytokine production in response to TLR and IL-1R signaling. Dysregulation of these pathways contributes to inflammatory conditions
LP-184 – Small Molecule
| Sponsor | Lantern Pharma |
| Indication | Triple Negative Breast Cancer |
| Phase | P-I |
| MOA | DNA synthesis inhibitors |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 3, 2024 |
- The US FDA has granted FTD to LP-184 for the treatment of Triple Negative Breast Cancer (TNBC) in patients, following the grant of FTD to treat Glioblastoma in Oct 2024
- The preclinical data showed tumor regression (107-141%) in both PARPi (PARP inhibitor) resistant & PARPi sensitive tumors across 10 TNBC PDX models. Recent preclinical data was presented at Immuno-Oncology Summit 2024
- LP-184 is being assessed in a P-Ia trial to evaluate its safety and tolerability in a broad range of solid tumor patients, incl. TNBC
- LP-184 leverages Lantern’s AI platform, RADR & is activated into its cytotoxic form by the enzymatic action of Prostaglandin Reductase 1 (PTGR1), to produce anti-tumor effects
CRB-701 – Biologic
| Sponsor | Corbus Pharma |
| Indication | R/R Metastatic Cervical Cancer |
| Phase | P-I |
| MOA | Anti Nectin 4 |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 9, 2024 |
- The US FDA has granted FTD to CRB-701 for the treatment of patients with r/r metastatic cervical cancer
- CRB-701 is being assessed in a 3-part, P-I trial evaluating its safety, PK & efficacy to treat advanced solid tumors linked to elevated Nectin-4. Enrollment of the dose escalation part has been completed which will be conducted in the US & Europe, initial data is expected to be reported in Q1’25
- CRB-701 (SYS6002) is an ADC with MMAE as the cytotoxic payload & site-specific, cleavable linker to target Nectin-4 expression on the cancer cells
LBT-SA7 – Vaccine
| Sponsor | LimmaTech Biologics |
| Indication | Skin And Soft Tissue Infections |
| Phase | P-I |
| MOA | Immunostimulants |
| RoA | Parenteral |
| Approval Authority | FDA |
| Date | Dec 19, 2024 |
- The US FDA has granted FTD to LBT-SA7 to avert skin and soft tissue infections caused due Staphylococcus aureus
- LBT-SA7 (multivalent toxoid vaccine) will be assessed in a P-I trial in the US, to demonstrate its safety & immunogenicity in 130 subjects (18 – 50yrs.), with initial results expected in H2’25

ATX101- Peptides
| Sponsor | Allay Therapeutics |
| Indication | Post-surgical Pain Following Total Knee Replacement Surgery |
| Phase | P-III (Planned) |
| MOA | Protein interaction domain and motif inhibitors |
| RoA | Subcutaneous, Intravenous |
| Approval Authority | FDA |
| Date | Dec 04, 2024 |
- The US FDA has granted BTD to ATX101 for treating adults with post-surgical pain following total knee replacement surgery
- Designation was based on dose-ranging P-II exploratory study of ATX101 vs bupivacaine (SoC) in 112 subjects, showing sustained pain relief for up to 2wks., decreased opioid use & associated side effects plus improved functional activities & satisfaction for up to 60 days. A registrational P-IIb trial in 200 individuals is planned in 2025 across the US
- ATX101 is an investigational formulation of bupivacaine (intracellular sodium ion channel blocker) and a biopolymer indicated for relieving pain post total knee arthroplasty (TKA)
Datopotamab Deruxtecan (Dato-DXd) – Biologic
| Sponsor | Daiichi Sankyo |
| Indication | EGFR-Mutated Non-Small Cell Lung Cancer |
| Phase | P-III |
| MOA | Anti-TROP2 ADC |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 09, 2024 |
- The US FDA has granted BTD to Dato-DXd for treating locally advanced or metastatic EGFR-mutated NSCLC in adults progressed on an EGFR tyrosine kinase inhibitor (TKI) and Pt-based CT
- Designation was based on P-II (TROPION-Lung05) & P-III (TROPION-Lung01) studies, with the results highlighted at ESMO 2024
- Daiichi Sankyo and AstraZeneca have filed a new BLA seeking accelerated approval for treating locally advanced or metastatic EGFR-mutated NSCLC in adults who received prior systemic therapies, incl. an EGFR-directed therapy
Tolebrutinib – Small Molecule
| Sponsor | Sanofi |
| Indication | Non-relapsing Secondary Progressive Multiple Sclerosis |
| Phase | P-III |
| MOA | BTK inhibitor |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 13, 2024 |
- The US FDA has granted BTD to tolebrutinib for treating adults with non-relapsing secondary progressive multiple sclerosis (nrSPMS)
- Designation was based on P-III (HERCULES) study assessing the safety & efficacy of tolebrutinib vs PBO to treat nrSPMS that showed a 31% delay in 6mos. confirmed disability progression onset, with 10% vs 5% having confirmed disability improvement
- Liver enzyme elevations (>3xULN) occurred in 4.1% vs 1.6%, with 0.5% showing ALT >20xULN within 90 days. Most cases resolved without intervention, and increased monitoring has mitigated serious liver issues
- Regulatory filings of tolebrutinib are being finalized in the US & prepared in the EU. In addition, the P-III (PERSEUS) study in primary progressive MS is underway, with results expected in H2’25
Jemperli – Biologic
| Sponsor | GSK |
| Indication | dMMR/MSI-H Rectal Cancer |
| Phase | P-II |
| MOA | PD-1-blocking antibody |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 16, 2024 |
- The US FDA has granted BTD to Jemperli for treating locally advanced dMMR)/MSI-H rectal cancer
- Designation was based on P-II study (conducted with Memorial Sloan Kettering Cancer Center) in dMMR rectal cancer patients (n=42), showing a 100% clinical CR in all, without any evidence of tumors on MRI, endoscopy, PET scan, or digital rectal exam
- Furthermore, sustained cCR was observed at a median follow-up of 26.3mos. in first 24 of them and is underway to assess the patients; safety profile aligned with known data without grade 3+ AEs. GSK’s registrational P-II (AZUR-1) trial is ongoing to validate this data
Trodelvy – Biologic
| Sponsor | Gilead |
| Indication | Extensive-Stage Small Cell Lung Cancer |
| Phase | P-II |
| MOA | DNA topoisomerase I inhibitors |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 17, 2024 |
- The US FDA has granted BTD to Trodelvy for treating adult with ES-SCLC whose disease has progressed on Pt-based CT
- The designation was based on P-II (TROPiCS-03) study ES-SCLC cohort, showing promising antitumor activity in both Pt-resistant (PR) & sensitive (PS) disease as a 2L treatment for ES-SCLC. Gilead plans to start a P-III trial
- P-III trials will assess Trodelvy alone and in combinations across various cancers, in collaboration with academic, industry & global partners
Sacituzumab Tirumotecan – Biologic
| Sponsor | Merck |
| Indication | EGFR Mutated Non-Squamous NSCLC |
| Phase | P-I/II |
| MOA | DNA topoisomerase I inhibitors |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 03, 2024 |
- The US FDA has granted BTD to sac-TMT (Kelun-Biotech holds the Greater China rights) for treating advanced or metastatic non-squamous EGFR-mutated (exon 19 deletion/exon 21 L858R) NSCLC in patients progressed post TKI & Pt-based CT
- Designation was supported by P-II expansion part of P-I/II study and two P-II trials of sac-TMT in EGFR-mutated NSCLC patients treated with at least two prior therapies, highlighted at ASCO 2023
- Merck is developing sac-TMT alone & combined with Keytruda under 10 global P-III trials for solid tumors, incl. TroFuse-004 (sac-TMT vs CT) & TroFuse-009 (sac-TMT vs doublet CT) in previously treated EGFR-mutated NSCLC
Zorevunersen – Antisense oligonucleotides
| Sponsor | Stoke Therapeutics |
| Indication | Dravet Syndrome |
| Phase | P-I/II |
| MOA | SCN1A gene targeting therapy |
| RoA | Intrathecal |
| Approval Authority | FDA |
| Date | Dec 04, 2024 |
- The US FDA has granted BTD to zorevunersen for treating Dravet syndrome with a confirmed mutation, not associated with gain-of-function, in the SCN1A gene
- The P-I/IIa and open label studies have depicted significant & sustained seizure reduction as well as improved cognition & behavior. The drug was well tolerated
- The Company is in ongoing talks with regulators about a P-III study of zorevunersen and will provide an update by YE’24
SER-155
| Sponsor | Seres Therapeutics |
| Indication | Bloodstream Infections |
| Phase | P-Ib |
| MOA | Bacteria replacements |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 09, 2024 |
- The US FDA has granted BTD to SER-155 for reducing bloodstream infections (BSIs) in adults, receiving allogeneic hematopoietic stem cell transplant (allo-HSCT) to treat hematological malignancies
- Designation was based on P-Ib study of SER-155 vs PBO, showing a 77% reduction in bacterial BSIs (10% vs 42.9%), shorter period of antibiotic use (9.2 vs 21.1 days) and reduced febrile neutropenia & gastrointestinal pathogen domination. Seres is looking for a collaboration to support its development
- SER-155 (oral) is a biotherapeutic aimed at preventing bacterial bloodstream infections and AMR-related outcomes in allo-HSCT patients by decolonizing GI pathogens and improving immune tolerance
Tobevibart – Biologic and Elebsiran – RNA Therapy
| Sponsor | Vir Biotechnology |
| Indication | Chronic Hepatitis Delta |
| Phase | P-II |
| MOA | Virus internalization inhibitors |
| RoA | Subcutaneous |
| Approval Authority | FDA & EMA |
| Date | Dec 12, 2024 |
- The US FDA & the EMA have granted BTD & PRIME Designation, respectively, to tobevibart & elebsiran for the treatment of chronic hepatitis delta (CHD) patients
- The designations were based on a P-II (SOLSTICE) trial evaluating tobevibart ± elebsiran in CHD patients with results presented at AASLD The Liver Meeting. The P-III (ECLIPSE) trial assessing tobevibart & elebsiran in CHD is to initiate in H1’25
Orpathys and Tagrisso – Small Molecules
| Sponsor | HUTCHMED |
| Indication | EGFR-Mutated NSCLC |
| Phase | P-III |
| MOA | Orpathys: MET tyrosine kinase inhibitor Tagrisso: irreversible EGFR TKI |
| RoA | Oral |
| Approval Authority | NMPA |
| Date | Dec 12, 2024 |
- The China’s NMPA has granted BTD to Orpathys (MET tyrosine kinase inhibitor) and Tagrisso (irreversible EGFR TKI) combination for treating locally advanced or metastatic EGFR+ NSCLC with MET amplification after progressing on EGFR inhibitor therapy
- The combination is being assessed under P-III (SACHI) study for its safety & efficacy in comparison with Pt-based doublet-CT (pemetrexed + cisplatin/carboplatin) to treat locally advanced or metastatic EGFR+ NSCLC
- The 1EP includes PFS by investigator evaluation while other EPs are PFS by IRC evaluation, OS, ORR, DoR, DCR, time to response (TTR) & safety

Imfinzi – Biologic
| Sponsor | AstraZeneca |
| Indication | Muscle-Invasive Bladder Cancer |
| Phase | P-III |
| MOA | PD-L1 inhibitor |
| RoA | Intravenous |
| Approval Authority | FDA |
| Date | Dec 06, 2024 |
- The US FDA has accepted & granted priority review to sBLA of Imfinzi for treating muscle-invasive bladder cancer (MIBC), with decision anticipated in Q2’25. Further submissions are under review across the EU, Japan & other regions
- sBLA was based on P-III (NIAGARA) study assessing perioperative Imfinzi + neoadj. CT followed by Imfinzi adj. vs CT alone treatment in patients (n=1,063) with MIBC, with data presented at ESMO 2024 & published in the NEJM
- Interim analysis showed reduced disease progression, recurrence, or death risk by 32% (mEFS: not reached vs 46.1mos.), 67.8% vs 59.8% were event free at 2yrs. Imfinzi also reduced death risk by 25% (mOS not reached), 82.2% vs 75.2% were alive at 2yrs.
Avutometinib + Defactinib
| Sponsor | Verastem Oncology |
| Indication | Recurrent KRAS Mutant Low-Grade Serous Ovarian Cancer |
| Phase | P-III |
| MOA | Avutometinib: RAF/MEK Clamp Defactinib: Selective FAK Inhibitor |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 30, 2024 |
- The US FDA has accepted & granted priority review (PDUFA: Jun 30, 2025) to NDA of avutometinib + defactinib for treatment-experienced KRAS-mutated recurrent LGSOC adults, under accelerated approval pathway. Launch is expected in mid-2025
- NDA was based on P-I (FRAME) trial & P-II (RAMP 201) trial of avutometinib (3.2mg, BIW) ± defactinib (200mg, BID) in LGSOC patients, identifying the combination as go-forward treatment in Part A; Parts B & C will assess the regimen’s safety & efficacy & Part D will explore low-dose regimen
- P-II showed significant ORRs & good tolerability. The P-III (RAMP 301) study will confirm results for this indication & is recruiting LGSOC patients regardless of KRAS mutations for indication expansion
Taletrectinib – Small Molecule
| Sponsor | Nuvation Bio |
| Indication | ROS1+ve NSCLC |
| Phase | P-II |
| MOA | ROS1 TKI |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 23, 2024 |
- The US FDA has accepted NDA of taletrectinib (next-generation ROS1 TKI) and granted priority for treating advanced ROS1+ NSCLC, with the decision anticipated on Jun 23, 2025
- Submission was based on pooled data from ongoing P-II [TRUST-I (China) & TRUST-II (global)] studies assessing the safety & efficacy of taletrectinib monotx. for treating advanced NSCLC, with the data highlighted at ESMO 2024
- Pooled analysis showed cORR of 89% & intracranial cORR of 77% with mDoR of 44mos. & mPFS of 46mos. in TKI-naïve patients (n=160). In TKI-pretreated patients (n=113), cORR was 56% & 62% for G2032R mutations, with intracranial cORR of 66%, mDoR of 17mos. & mPFS of 10mos.

101-PGC-005 – Antivirals
| Sponsor | PIF Partners |
| Indication | Systemic Juvenile Idiopathic Arthritis (sJIA) Flares |
| Phase | P-III |
| MOA | Glucocorticoid receptor agonists |
| RoA | Unknown |
| Approval Authority | FDA |
| Date | Dec 03, 2024 |
- The US FDA has granted RPDD to 101-PGC-005 (‘005) for treating systemic juvenile idiopathic arthritis (sJIA) flares
- 101-PGC-005 (Type IA prodrug of dexamethasone targeting CD206+ macrophages) is currently being investigated under P-III study to treat ARDS induced by COVID-19 across 9 sites in India
NB-001
| Sponsor | Nobias Therapeutics |
| Indication | DiGeorge syndrome (22q11.2 Deletion Syndrome) |
| Phase | P-II |
| MOA | mGluR Modulator |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 05, 2024 |
- The US FDA has granted ODD & RPDD to NB-001 for the treatment of patients with neuropsychiatric symptoms related to 22q11.2 deletion syndrome (DiGeorge syndrome/22q11DS)
- NB-001 is currently under investigation in a P-II trial assessing NB-001 vs PBO in 22q11DS patients with top-line results presented at CNS Annual Meeting, 2023
- NB-001 (mGluR modulator) disrupts neuronal signaling that relieves neuropsychiatric symptoms
THIO – Small Molecule
| Sponsor | MAIA Biotechnology |
| Indication | Pediatric-Type Diffuse High-grade Gliomas |
| Phase | P-II |
| MOA | Telomere targeting agent |
| RoA | Unknown |
| Approval Authority | FDA |
| Date | Dec 16, 2024 |
- The US FDA has granted RPDD to THIO for treating pediatric type diffuse high-grade gliomas (PDHGG)
- Alongside RPDD, the drug has also been designated with ODD for hepatocellular carcinoma (HCC), small cell lung cancer (SCLC) and glioblastoma
- THIO (6-thio-dG or 6-thio-2’-deoxyguanosine) is a telomere-targeting candidate that is being investigated for NSCLC
HORA-PDE6b – Gene Therapy
| Sponsor | EyeDNA Therapeutics (Coave’s Subsidiary) |
| Indication | Retinal Dystrophy |
| Phase | P-I/II |
| MOA | Gene transference |
| RoA | Subretinal |
| Approval Authority | FDA |
| Date | Dec 17, 2024 |
- The US FDA has granted RPDD to HORA-PDE6b for treating inherited retinal dystrophy (IRD) caused by PDE6b gene mutations
- HORA-PDE6b is being studied under P-I/II trial for its safety & efficacy, with the 24mos. follow-up data highlighted at ARVO 2024
- The company is seeking for accelerated approval across the US & the EU for patients with this indication
- HORA-PDE6b, an AAV5-based gene therapy, delivers a functional PDE6b gene to the subretinal space, restoring protein synthesis in photoreceptors and potentially halting retinal degeneration in PDE6b-deficient patients
Relutrigine – Small Molecule
| Sponsor | Praxis Precision Medicines |
| Indication | Dravet Syndrome |
| Phase | P-II |
| MOA | Sodium channel antagonists |
| RoA | Oral |
| Approval Authority | FDA |
| Date | Dec 18, 2024 |
- The US FDA has granted RPDD to relutrigine for treating Dravet syndrome
- Relutrigine is being assessed under P-II (EMBOLD) study for its safety & efficacy in reducing seizures. Findings from SCN2A and SCN8A patients in cohort 1 were highlighted at AES 2024
- Study depicted a 46% reduction in PBO-adjusted monthly motor seizure during double-blind, 30% were seizure free, 77% reduction in median seizure rate during long-term extension & notable improvements in alertness, communication & seizure severity
- The company has begun recruitment for second, registrational cohort for SCN2A and SCN8A patients, with topline data anticipated in H1’26
Manufactured Red Blood Cells
| Sponsor | Safi Biotherapeutics |
| Indication | Sickle Cell Disease |
| Phase | Preclinical |
| MOA | Unkown |
| RoA | Unkown |
| Approval Authority | FDA |
| Date | Dec 18, 2024 |
- The US FDA has granted ODD & RPDD to manufactured RBCs (mRBCs) for use in chronic transfusion to treat sickle cell patients
- The company is advancing its validation of mRBCs cGMP manufacturing for clinical studies & anticipates initiating them by 2027, post-completion of IND-enabling activities

Magnetic EEG Resonance Therapy (MeRT) System
| Sponsor | Wave Neuroscience |
| Indication | Post-Traumatic Stress Disorder |
| Phase | N/A |
| MOA | N/A |
| RoA | N/A |
| Approval Authority | FDA |
| Date | Dec 03, 2024 |
- The US FDA has granted BDD to Magnetic EEG Resonance Therapy (MeRT) system as an adjunctive treatment of post-traumatic stress disorder (PTSD)
- The MeRT system uses a proprietary algorithm to personalize Transcranial Magnetic Stimulation (TMS) treatment based on an individual’s brain wave data
FieldForce Ablation System
| Sponsor | Field Medical |
| Indication | Ventricular Tachycardia |
| Phase | N/A |
| MOA | N/A |
| RoA | N/A |
| Approval Authority | FDA |
| Date | Dec 05, 2024 |
- The US FDA has granted BDD to FieldForce Ablation System for sustained monomorphic scar-related ventricular tachycardia (VT), which makes it eligible for Total Product Life Cycle Advisory Program (TAP) Pilot
- The FieldForce Ablation System consists of a single-point contact force PFA catheter with proprietary FieldBending tech, enabling precise targeted lesions and large-volume transmural lesions in the ventricle
MeMed Severity Test
| Sponsor | MeMed |
| Indication | Suspected Sepsis |
| Phase | N/A |
| MOA | N/A |
| RoA | N/A |
| Approval Authority | FDA |
| Date | Dec 10, 2024 |
- The US FDA has granted BDD to MeMed Severity test to manage suspected acute infections and suspected sepsis
- The test assesses various proteins in the blood sample using advanced host-response technology and identifies the risk of suspected acute infection deteriorating to severe outcomes within 72hrs. or death within 14 days through ML

Tobevibart and Elebsiran
| Sponsor | Vir Biotechnology |
| Indication | Chronic Hepatitis Delta |
| Phase | P-II |
| MOA | Virus internalization inhibitors |
| RoA | Subcutaneous |
| Approval Authority | EMA |
| Date | Dec 12, 2024 |
- The US FDA & the EMA have granted BTD & PRIME Designation, respectively, to tobevibart & elebsiran for the treatment of chronic hepatitis delta (CHD) patients
- The designations were based on a P-II (SOLSTICE) trial evaluating tobevibart ± elebsiran in CHD patients with results highlighted at AASLD The Liver Meeting. The P-III (ECLIPSE) trial assessing tobevibart & elebsiran in CHD is to initiate in H1’25
GSK’227 – Biologic
| Sponsor | GSK |
| Indication | Relapsed Extensive-stage Small-cell Lung Cancer |
| Phase | P-I |
| MOA | B7-H3-targeting ADC |
| RoA | Intravenous |
| Approval Authority | EMA |
| Date | Dec 16, 2024 |
- The EMA has granted PRIME designation to GSK5764227 (GSK’227 or HS-20093), B7-H3-targeted ADC, for treating relapsed extensive-stage SCLC based on preliminary data from P-I (ARTEMIS-001) study (carried out by Hansoh Pharma)
- The P-I trial is assessing safety, tolerability & anti-tumor activity of GSK’227 in locally advanced or metastatic solid tumors (incl. ES-SCLC) patients (n=>200), with data highlighted at WCLC 2024. A global P-I trial has begun to support its registrational filing
- GSK secured GSK’227’s exclusive worldwide rights (excl. mainland mainland China, Hong Kong, Macau & Taiwan) from Hansoh as per an agreement b/w them

AAV2-hAQP1 – Gene Therapy
| Sponsor | MeiraGTx Holdings |
| Indication | Grade 2/3 Radiation-Induced Xerostomia |
| Phase | P-II |
| MOA | Gene transference |
| RoA | Undisclosed |
| Approval Authority | FDA |
| Date | Dec 09, 2024 |
- The US FDA has granted RMAT designation to AAV2-hAQP1 for treating Grade 2/3 radiation-induced xerostomia (RIX)
- Designation was based on P-I (AQUAx) study showing significantly improved patient-reported outcomes & saliva production without treatment-related SAEs or DLTs. Results were highlighted at AAOM 2024
- The P-II (AQUAx2) trial currently assesses AAV2-hAQP1 vs PBO continues recruitment and dosing across the US, UK & Canada
References
- Businesswire
- PRWeb
- Globe Newswire
- PR Newswire
- EIN Presswire
- Google News
Related Post: New Drug Designations – November 2024



