The US FDA New Drug Approvals in March 2025
Shots:
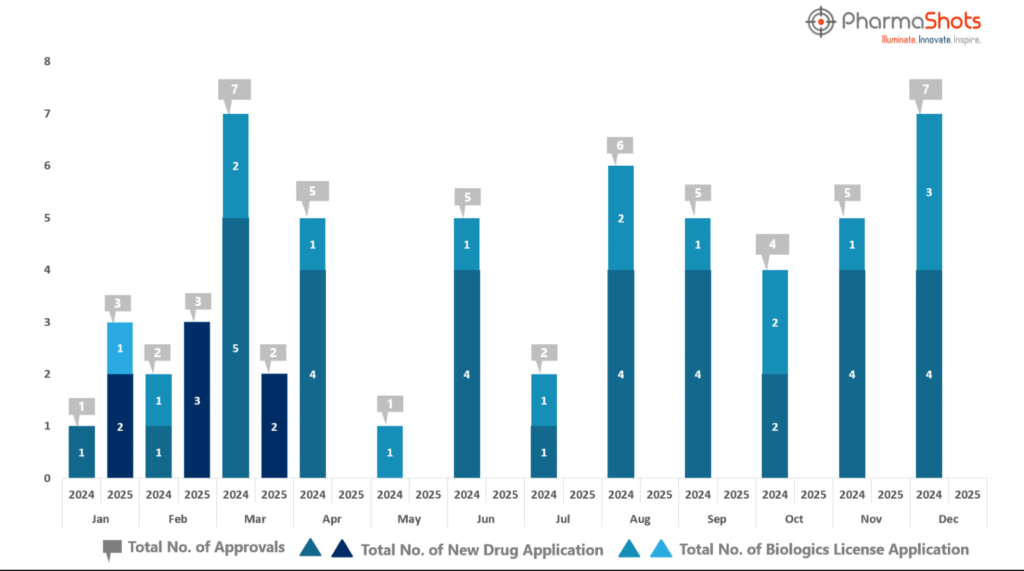
- PharmaShots has compiled a list of US FDA-approved drugs in the month of March 2025
- The US FDA has approved a total of 2 new drug including 2 new molecular entities leading to the treatment of patients and advances in the healthcare industry
- The major highlighted drug was GSK’s Blujepa securing approval for treating Uncomplicated Urinary Tract Infections (uUTIs)
Company: GSK
Product: Blujepa
Active Ingredient: Gepotidacin
Disease: Uncomplicated Urinary Tract Infections (uUTIs)
Date: Mar 25, 2025
Shots:
- The US FDA has approved Blujepa to treat uUTIs in female adults (≥40kg) & adolescents (≥12yrs., ≥40kg) caused by E. coli, K. pneumoniae, C. freundii complex, S. saprophyticus & E. faecalis; commercially available in H2’25
- Approval was based on P-III (EAGLE-2, n=1531; EAGLE-3, n=1605) studies assessing gepotidacin (1500mg, PO, BID for 5 days) vs nitrofurantoin (100mg, PO, BID for 5 days) to treat uUTIs with the follow-up of 28 days
- Studies depicted non-inferiority of gepotidacin to nitrofurantoin (SoC for uUTI), with the success rates of 50.6% (162/320) vs 47% (135/287; treatment difference: 4.3%) in EAGLE-2 & 58.5% (162/277) vs 43.6% (115/264; treatment difference: 14.6%) in EAGLE-3
Company: Alnylam Pharmaceuticals and Sanofi
Product: Qfitlia
Active Ingredient: Fitusiran
Disease: Hemophilia A or B
Date: Mar 28, 2025
Shots:
- The US FDA has approved Qfitlia to treat routine prophylaxis and prevent or reduce the frequency of bleeding episodes in pts. (age≥ 12yrs.) with hemophilia A or B, with or without factor VIII or IX inhibitors. Regulatory submissions have been completed in China & Brazil
- Qfitlia published clinical data in the NEJM in 2017, showing a reduction in bleeding rates in haemophilia pts. and initiating the P-III development program
- In 2014, Sanofi gained global rights to co-develop & co-commercialize Qfitlia under a license & collaboration agreement which was later upgraded to full global rights in 2018, while Alnylam became eligible for tiered royalties of 15-30% on global net sales
Related Post: Insights+: The US FDA New Drug Approvals in February 2025



