PharmaShots Weekly Snapshots (Oct 13, 2025 – Oct 17, 2025)
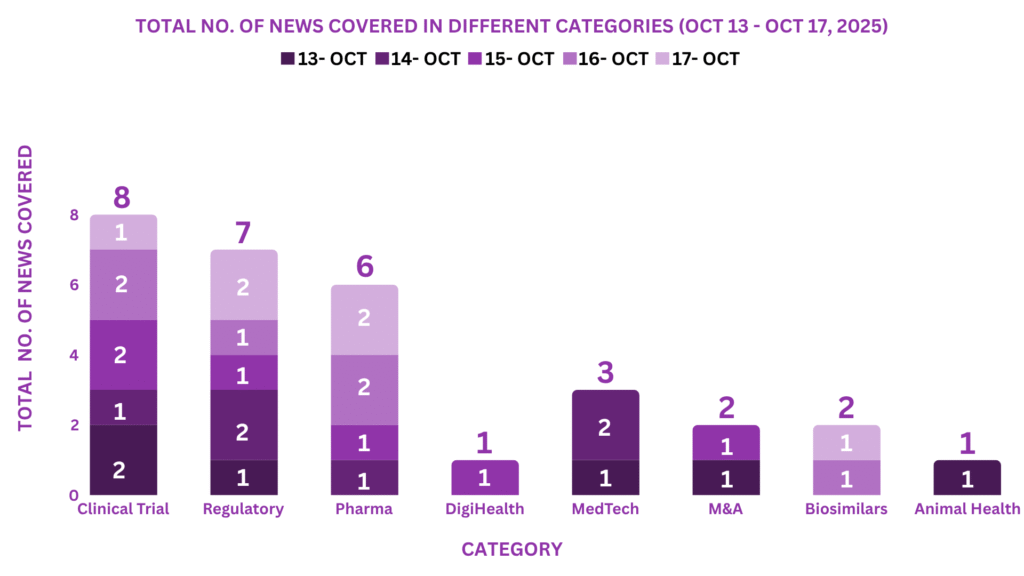
This week, PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, DigiHealth, Animal Health, and Biosimilars. Check out our full report below:

Arcus Biosciences Reports the P-II (EDGE-Gastric) Trial Data on Domvanalimab + Yutuo (Zimberelimab) for Gastroesophageal Adenocarcinomas
Read More: Arcus Biosciences
HUTCHMED Reports the P-II/III (FRUSICA-2) Trial Data of Fruquintinib + Sintilimab for Renal Cell Carcinoma (RCC)
Read More: HUTCHMED
SystImmune Doses First Patient in P-II/III (IZABRIGHT-Breast01) Trial of Iza-Bren to Treat Triple Negative Breast Cancer (TNBC)
Read More: SystImmune
Boehringer Ingelheim and Click Therapeutics Report P-III (CONVOKE) Trial Data on CT-155 for Schizophrenia
Read More: BI and Click Therapeutics
Pfizer Reports Topline P-III (HER2CLIMB-05) Trial Data of Tukysa (Tucatinib) in Metastatic Breast Cancer (MBC)
Read More: Pfizer
Novartis Highlights P-III (APPLAUSE-IgAN) Trial Findings on Fabhalta (Iptacopan) for IgA Nephropathy
Read More: Novartis
Eli Lilly Reports Topline Data from P-III (ACHIEVE-2 & 5) Trials of Orforglipron for Type 2 Diabetes
Read More: Eli Lilly
Merck Highlights P-III (KEYNOTE-B96/ENGOT-ov65) Trial Findings on Keytruda for Platinum-Resistant Ovarian Cancer
Read More: Merck

BeOne Medicines’ Sonrotoclax Receives FDA’s Breakthrough Therapy Designation for R/R Mantle Cell Lymphoma (MCL)
Read More: BeOne Medicines
MannKind Reports the US FDA’s sBLA Acceptance of Afrezza (Inhaled Insulin) for Children and Adolescents with Diabetes
Read More: MannKind
China’s NMPA Approves GSK’s Shingrix to Protect Individuals Against Shingles
Read More: GSK
Corcept Therapeutics Reports the EMA’s MAA Submission of Relacorilant to Treat Platinum-Resistant Ovarian Cancer
Read More: Corcept Therapeutics
Incyte Reports the Health Canada’s Approval of Opzelura (Ruxolitinib) for Atopic Dermatitis (AD)
Read More: Incyte
LEO Pharma Reports the NMPA’s NDA Acceptance for Anzupgo (Delgocitinib) to Treat Chronic Hand Eczema (CHE)
Read More: LEO Pharma
Johnson & Johnson Reports the US FDA’s sNDA Acceptance & Priority Review of Akeega to Treat Metastatic Hormone-Sensitive Prostate Cancer (mHSPC)
Read More: J&J

Nabla Bio Collaborates with Takeda to Advance AI-Driven Protein Therapeutics Design
Read More: Nabla Bio and Takeda
insitro and Bristol Myers Squibb Collaborate to Advance Drug Discovery for Amyotrophic Lateral Sclerosis (ALS)
Read More: insitro and BMS
Novo Nordisk Enters ~$2.1B Deal with Omeros to Advance Zaltenibart for Rare Blood & Kidney Disorders
Read More: Novo Nordisk and Omeros
Boehringer Ingelheim and AimedBio Partner to Advance an ADC Therapeutic in Oncology
Read more: BI and AimedBio
EVOQ Therapeutics Signs a ~$500M Deal with Sanofi for NanoDisc Technology
Read More: EVOQ Therapeutics and Sanofi
Leads Biolabs and Dianthus Therapeutics Sign ~$1B Licensing Deal to Jointly Advance LBL-047
Read More: Leads Biolabs and Dianthus Therapeutics

Surgical Theater’s SyncAR Spine Platform Secures the US FDA’s 510(k) Clearance for Spine Surgery
Read More: Surgical Theater
NEXTBIOMEDICAL Enrolls First Patient in RESORB Trial of Nexsphere-F to Use it in Genicular Artery Embolization (GAE) for Knee Osteoarthritis
Read More: NEXTBIOMEDICAL
Roche’s Elecsys pTau181 Test Receives the US FDA’s Clearance to Rule Out Alzheimer’s Disease
Read More: Roche

BMS to Strengthen its Cell Therapy Portfolio with $1.5B Orbital Therapeutics Acquisition
Read More: BMS and Orbital Therapeutics
BioCryst to Acquire Astria Therapeutics in ~$700M Cash-and-Stock Transaction
Read More: BioCryst and Astria Therapeutics

Biocon Biologics and Civica Partner to Launch Private-Label Insulin Glargine in the US
Read More: Biocon Biologics and Civica
FDA Approves Celltrion’s Yuflyma (Biosimilar, Humira) and its Unbranded Version for Hidradenitis Suppurativa (HS) and Uveitis in Younger Patients
Read More: Celltrion

Vara Reports CE Mark Approval of its Breast Imaging AI for Independent Second Reading of Mammography
Read More: Vara

Zoetis’ Lenivia (Izenivetmab) Receives the CVMP’s Recommendation for Osteoarthritis Pain Relief in Dogs
Read More: Zoetis
Related Post: PharmaShots Weekly Snapshots (Oct 06, 2025 – Oct 10, 2025)

