New Drug Designations – August 2025
Shots:
- PharmaShots’ Designation Report provides a concise overview of the latest drug designations granted by major regulatory authorities, including the FDA, EMA, MHLW, Health Canada, and NMPA
- The August 2025 report covers designations granted to 37 drugs and 4 medical devices, spanning 14 small molecules, 5 biologics, 7 cell and gene therapies & 4 medical devices, among others
- Significant trends this month show that Clarametyx Biosciences’ CMTX-101 secured Fast Track and Qualified Infectious Disease Product Designations for the treatment of chronic bacterial pulmonary infections linked with cystic fibrosis
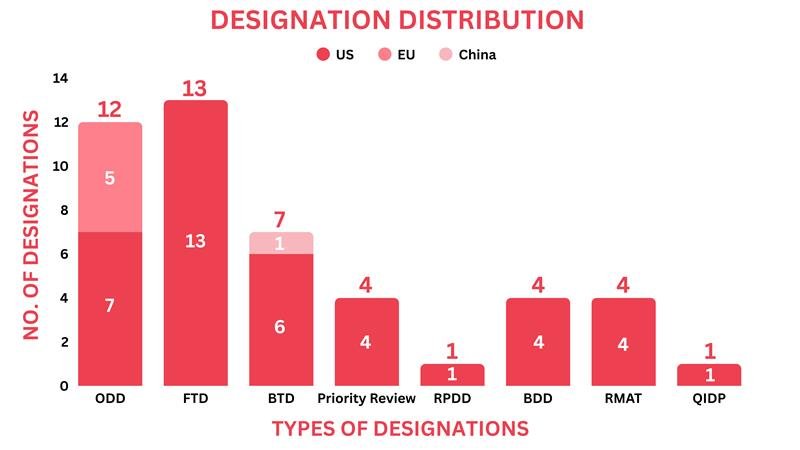

Drugs receiving orphan drug designation by global regulatory bodies echoed as small molecules, peptides, and antisense oligonucleotides. Around 12 drugs received the orphan drug designation across multiple indications.
A Quick Look
Small Molecules: Atumelnant (Crinetics Pharmaceuticals) for Congenital Adrenal Hyperplasia, D3S-001 (D3 Bio) for KRAS G12C-mutated colorectal cancer, INV043 (Invion) for Anal Cancer,, Rilzabrutinib (Sanofi) for IgG4-related disease, Alpibectir (BioVersys) for Tuberculosis, RCT002 (Roca Therapeutics) for Radiation Maculopathy, ADX-2191 (Aldeyra Therapeutics) for Primary Vitreoretinal Lymphoma, Peptide: Dusquetide (Soligenix) for Behçet’s Disease, RLS-0071 (ReAlta Life Sciences) for Graft-Versus-Host Disease, Antisense Oligonucleotide: ART4 (Arnatar Therapeutics) for Alagille Syndrome, VGT-1849B (Vanda Pharmaceuticals) for Polycythemia Vera, Recombinant Fusion Protein: KER-065 (Keros Therapeutics) for Duchenne muscular dystrophy (DMD)

Around 12 drugs received the Fast Track Designation as gene therapy, small molecules, biologics, peptides, & proteins.
A Quick Look
Gene Therapy: AAVB-039 (AAVantgarde Bio) for Stargardt disease, Small Molecule: ADX-2191 (Aldeyra Therapeutics) for Retinitis pigmentosa, Birelentinib (Dizal Pharma) for R/R Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma, HLD-0915 (Halda Therapeutics) for Metastatic Castration-Resistant Prostate Cancer, CS1 (Cereno Scientific) for Pulmonary arterial hypertension, Stenoparib (Allarity Therapeutics) for Ovarian Cancer, TT125-802 (TOLREMO Therapeutics) for EGFRm & KRAS G12C-mutated NSCLC, NRX-100 (NRx Pharmaceuticals) for Suicidal ideation, Peptide Drug Conjugates: PQ203 (ProteinQure) for Triple-Negative Breast Cancer, Biologic: CMTX-101 (Clarametyx Biosciences) for Chronic bacterial pulmonary infections in Cystic Fibrosis, Peptide: Pemvidutide (Altimmune) for Alcohol Use Disorder, Protein: Gelsolin (BioAegis Therapeutics) for Decompression sickness

Regulatory bodies designated seven drugs with Breakthrough Therapy Designation, ranging from Peptide and Antisense oligonucleotides to biologics.
A Quick Look
Small Molecule: D3S-001 (D3 Bio) for KRAS G12C-mutated NSCLC, Peptide: Rusfertide (Protagonist Therapeutics) for Erythrocytosis in Polycythemia Vera, Antisense Oligonucleotide: DYNE-251 (Dyne Therapeutics) for Duchenne muscular dystrophy, Biologic: Izalontamab brengitecan (SystImmune) for EGFRm NSCLC, Rinatabart sesutecan (Genmab) for Endometrial cancer, Ifinatamab deruxtecan (Daiichi Sankyo & Merck) for Extensive-stage small cell lung cancer, ATG-022 (Antengene) for G/GEJ Cancers

Four drugs, ranging from small molecules to peptide, were given priority review.
A Quick Look
Small Molecules: Blujepa (GSK) for Uncomplicated Urogenital Gonorrhoea, Peptide: Setmelanotide (Rhythm Pharmaceuticals) for Acquired Hypothalamic Obesity, Cell Therapy: Omidubicel (Ayrmid) for Aplastic Anemia, Breyanzi (BMS) for R/R Marginal Zone Lymphoma

One drug received the rare pediatric disease designation as an antisense oligonucleotide
A Quick Look
Antisense Oligonucleotide: ART4 (Arnatar Therapeutics) for Alagille Syndrome

Four devices received the breakthrough device designation by the US FDA.
A Quick Look
Kidney Preservation Device (BMI OrganBank) for Kidney Transplant, Triojection (SpinaFX Medical) for Lumbar disc herniations, Haystack MRD Circulating Tumor DNA Test (Quest Diagnostics) for Colorectal cancer, NucleoCapture (Santersus) for Systemic Lupus Erythematosus

A single drug received the qualified infectious disease product designation
A Quick Look
Biologic: CMTX-101 (Clarametyx Biosciences) for Chronic bacterial pulmonary infections in Cystic Fibrosis

Four drugs received the regenerative medicine advanced therapy designation.
A Quick Look
Cell Therapy: GLPG5101 (Galapagos) for Mantle Cell Lymphoma, BEAM-101 (Beam Therapeutics) for Sickle Cell Disease, HB-adMSCs (Hope Biosciences) for Relapsing-Remitting Multiple Sclerosis, Gene Therapy: VG801 (VeonGen Therapeutics) for Stargardt Disease
Related Post: New Drug Designations: July 2025



