PharmaShots Weekly Snapshots (Sep 15, 2025 – Sep 19, 2025)
PharmaShots Weekly Snapshots (Sep 15, 2025 – Sep 19, 2025)
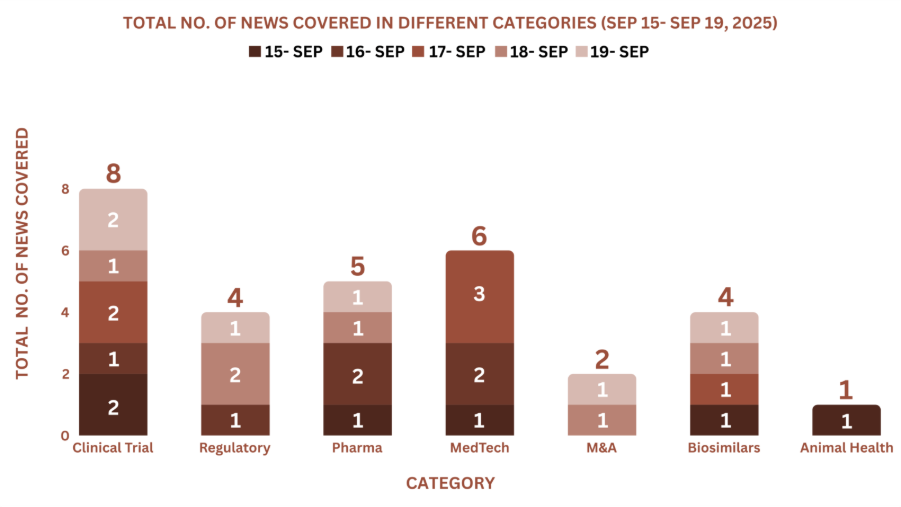
This week, PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, COVID-19, M&A and Biosimilars. Check out our full report below:

MAIA Biotechnology Reports P-II (THIO-101) Trial Data of Ateganosine Regimen for Advanced NSCLC
Read More: MAIA Biotechnology
Akeso Reports First Patient Dosing in P-II (COMPASSION-36) Trial of Cadonilimab for PD-1 Treatment-Resistant Hepatocellular Carcinoma (HCC)
Read More: Akeso
Areteia Therapeutics Reports P-III (EXHALE-4) Trial Data of Dexpramipexole to Treat Eosinophilic Asthma
Read More: Areteia Therapeutics
Novo Nordisk Reveals P-III (REDEFINE 1) Trial Data of Cagrilintide for Weight Loss in Obese/Overweight
Read More: Novo Nordisk
Sanofi Reports P-IIa (HS-OBTAIN) Trial Data of Brivekimig for Hidradenitis Suppurativa
Read More: Sanofi
AstraZeneca Reports Interim P-III (TULIP-SC) Trial Data of Saphnelo for Systemic Lupus Erythematosus (SLE)
Read More: AstraZeneca
LEO Pharma Reports Data from P-III Study of Delgocitinib Cream in Adolescents with Moderate to Severe Chronic Hand Eczema (CHE)
Read More: LEO Pharma
Intellia Therapeutics Completes Enrollment in the P-III (HAELO) Study of Lonvoguran Ziclumeran (lonvo-z) in Hereditary Angioedema (HAE) Patients
Read More: Intellia Therapeutics

Ionis’ ION582 Receives the US FDA’s Breakthrough Therapy Designation to Treat Angelman Syndrome
Read More: Ionis
Merck and Daiichi Sankyo’s Raludotatug deruxtecan Receives FDA’s Breakthrough Therapy Designation for Ovarian, Primary Peritoneal, or Fallopian Tube Cancers
Read More: Merck and Daiichi Sankyo
Deciphera Pharmaceuticals Reports the EC’s Approval of Romvimza for Symptomatic Tenosynovial Giant Cell Tumor (TGCT)
Read More: Deciphera Pharmaceuticals
Biogen’s Zurzuvae Receives the EC’s Approval to Treat Severe Postpartum Depression
Read More: Biogen
Incyte Reports the US FDA’s sNDA Approval of Opzelura (Ruxolitinib) for Atopic Dermatitis (AD)
Read More: Incyte

Ocugen Joins Forces with Kwangdong Pharmaceutical for OCU400 to Treat Retinitis Pigmentosa
Read More: Ocugen and Kwangdong Pharmaceutical
Monte Rosa Therapeutics Enters a ~$5.7B with Novartis to Develop Degraders for Immune-Mediated Diseases
Read More: Monte Rosa Therapeutics and Novartis
VarmX Inks a ~$2.2B Strategic Collaboration and Option Agreement with CSL for VMX-C001 to Advance Coagulation Treatment
Read More: VarmX and CSL
VectorY Therapeutics Collaborates with Shape Therapeutics to Develop Vectorized Antibodies for Neurodegenerative Diseases
Read More: VectorY Therapeutics and Shape Therapeutics
Avant Technologies and Austrianova Form Joint Venture Klothonova to Develop Klotho-Based Cell Therapies
Read More: Avant Technologies and Austrianova

AVITA Medical Secures European CE Mark Approval for Recell Go to Advance Burn and Wound Healing
Read More: AVITA Medical
Novocure Reports MHLW’s Approval of Optune Lua for NSCLC
Read More: Novocure
Womed Reports the US FDA’s Approval of Womed Leaf to Treat Asherman Syndrome
Read More: Womed
Biocartis Reports the US FDA’s Approval of Idylla CDx MSI Test to Identify MSI-H Colorectal Cancer Patients
Read More: Biocartis
Johnson & Johnson MedTech Launches Shockwave Javelin Peripheral IVL Catheter in the EU
Read More: Johnson & Johnson
Amber Implants Reports the US FDA’s 510(k) Clearance of VCFix Spinal System for Treating Vertebral Compression Fractures
Read More: Amber Implants

Roche to Acquire 89bio for ~$3.5B, Strengthening its CVRM Portfolio
Read More: Roche and 89bio
Biogen to Acquire Alcyone Therapeutics to expand its drug delivery solutions Portfolio
Read More: Biogen & Alcyone Therapeutics

Bio-Thera Solutions Collaborates with Jamjoom Pharmaceuticals to Commercialize BAT2306 (Biosimilar, Cosentyx)
Read More: Bio-Thera Solutions and Jamjoom Pharmaceuticals
Alteogen’s Eyluxvi (Biosimilar, Eylea) Receives the EC’s Approval for Retinal Diseases
Read More: Alteogen
Biocon Biologics Receives the FDA’s Approval & Provisional Interchangeability Designation for Aukelso and Bosaya (Biosimilars, Xgeva and Prolia)
Read More: Biocon Biologics
Celltrion Receives the Health Canada’s Approval for Stoboclo and Osenvelt (Biosimilar, Prolia & Xgeva)
Read More: Celltrion

Zoetis’ Portela (Relfovetmab) Receives the CVMP’s Positive Opinion for Osteoarthritis Pain Relief in Cats
Read More: Zoetis
Related Post: PharmaShots Weekly Snapshots (Sep 08, 2025 – Sep 12, 2025)

