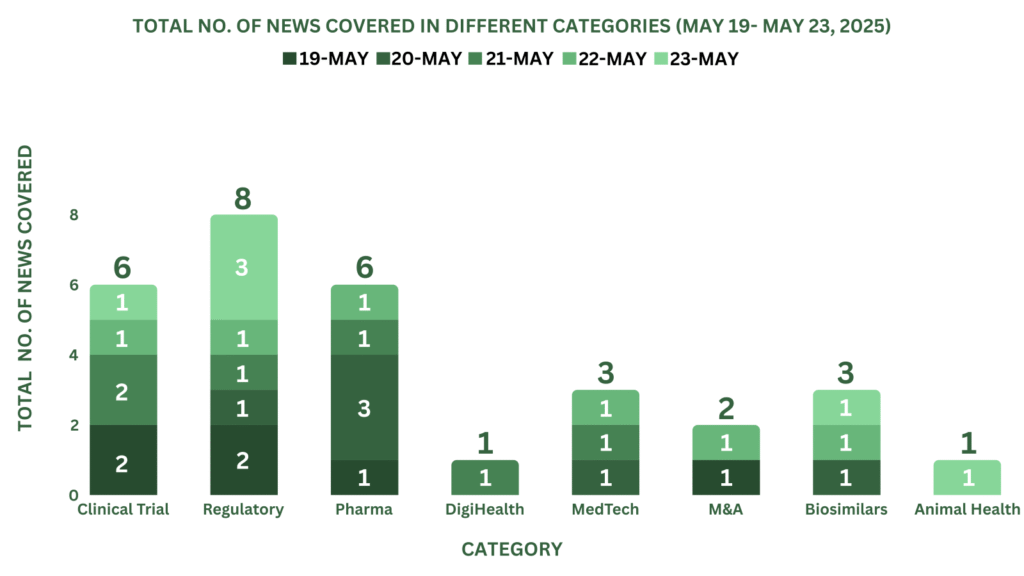
PharmaShots Weekly Snapshots (May 19, 2025 – May 23, 2025)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Biosimilar, DigiHealth & Animal Health. Check out our full report below:

Apnimed Reports Topline P-III (SynAIRgy) Trial Data of AD109 for Obstructive Sleep Apnea (OSA)
Read More: Apnimed
Ionis Reveals Topline P-III (ESSENCE) Trial Data of Olezarsen for Moderate Hypertriglyceridemia
Read More: Ionis
Viridian Therapeutics Releases the Long-Term Durability Data Evaluating Veligrotug in P-III (THRIVE) Study in Active TED
Read More: Virdian
KaliVir Immunotherapeutics Reports Completion of First Cohort of P-I/Ib (STEALTH-001) Study in Advanced Solid Tumors
Read More: KaliVir
Merck Reports P-II (WILLOW) Trial Data of Enpatoran for Cutaneous Lupus Erythematosus (CLE) and Systemic Lupus Erythematosus (SLE)
Read More: Merck
Gyre Therapeutics Releases P-III Trial Findings on Hydronidone for Chronic Hepatitis B-Associated Liver Fibrosis
Read More: Gyre Therapeutics

BMS’ Opdivo Receives the EC’s Approval as a Perioperative Treatment of Resectable NSCLC
Read More: BMS
GSK Receives the MHLW’s Approval for Blenrep (Belantamab Mafodotin) Regimens to Treat R/R Multiple Myeloma
Read More: GSK
Novavax’s Nuvaxovid Receives the US FDA Approval for Active Immunization against COVID-19
Read More: Novavax
China’s NMPA Grants Approval to InnoCare Pharma’s Minjuvi + Lenalidomide to Treat R/R DLBCL
Read More: Innocare
AstraZeneca’s Tagrisso (Osimertinib) Secures Health Canada’s Conditional Approval to Treat Unresectable EGFR-Mutated NSCLC
Read More: Astrazeneca
GSK Reports the US FDA’s Approval of Nucala (Mepolizumab) for Chronic Obstructive Pulmonary Disease (COPD)
Read More: GSK
Roche Reports the US FDA’s Approval of Susvimo to Treat Diabetic Retinopathy
Read More: Roche
The US FDA Approves Arcutis Biotherapeutics’ Zoryve Topical Foam for Plaque Psoriasis
Read More: Arcutis Biotherapeutics

Skye Bioscience Enters a Formulation Development Agreement with Arecor Therapeutics for Nimacimab
Read More: Skye
Regeneron to Acquire 23andMe Assets for $256M
Read More: Regeneron
CRISPR Therapeutics Enters a Multi-Target Collaboration with Sirius Therapeutics to Develop Novel siRNA Therapies
Read More: Crispr
VantAI and Blueprint Medicines Expands License Agreement to Advance Induced Proximity Drug Discovery
Read More: Vant AI and Blueprint
Genentech Collaborates with Orionis to Identify and Develop Molecular Glue Therapies for Cancer Targets
Read More: Genentech
Pfizer Enters a ~$6.05B Exclusive Licensing Agreement with 3SBio for SSGJ-707
Read More: Pfizer

The US FDA Grants 510(k) clearance to Stryker’s OptaBlate BVN System for Vertebrogenic Pain
Read More: Stryker
ReGelTec’s HYDRAFIL System Gains European CE Mark Approval for Chronic Low Back Pain
Read More: Regeltecs
RamSoft and Therapixel Form a Commercial Partnership For an Integrated AI Breast Imaging Solution
Read More: Ramsoft

BioMarin Pharmaceutical to Acquire Inozyme Pharma for ~$270M, Strengthening its Enzyme Therapies Portfolio
Read More: BioMarin Pharmaceutical and Inozyme Pharma
Sanofi to Acquire Vigil Neuroscience for ~$470M
Read More: Sanofi and Vigil Neuroscience

Fresenius Kabi’s Otulfi (Biosimilar, Stelara) Receives the US FDA’s Interchangeability Designation
Read More: Fresenius
Celltrion’s Yuflyma (Biosimilar, Humira) Receives the US FDA’s Interchangeability Designation for All Presentations
Read More: Celltrion
Sandoz Launches Pyzchiva Autoinjector (Biosimilar, Stelara) in the EU for Chronic Inflammatory Diseases
Read More: Sandoz

Petvivo Holdings Collaborates with PiezoBioMembrane to Develop Functional Biomaterials
Read More: Petvivo Holdings and PiezoBioMembrane

Viz.ai Enters a Multi-Year Partnership with Sanofi and Regeneron to Improve Management and Care of COPD
Read More: Viz AI
Related Post: PharmaShots Weekly Snapshots (May 12, 2025 – May 16, 2025)

