The Emergence of Artificial Intelligence in the Pharmaceutical Industry
The dependability of digital technology has augmented through a variety of industries, including communication, transportation, manufacturing, medical, and pharmaceutical companies. Development in technologies has made it possible to merge medical science with digital platforms, allowing for the individualised delivery of services to a large population, making therapy more effective and convenient. Today, technology has become an integral part of medical science and is being employed in a variety of ways by clinicians and pharmacists. Many pharmaceutical manufacturing companies have adopted digital technology into their working procedure as it brings numerous benefits, such as a more efficient and organised workflow. Previously, pharmacists faced numerous obstacles in the workplace, which have now been eliminated with the help of technologies. With various different digital technologies available in the market, one which is being avidly used in the pharmaceutical sector is Artificial Intelligence.[1]
What is Artificial Intelligence?
Artificial Intelligence (AI) is an integration of machine and human intelligence with its primary objective being to recognise problems and process them using relevant information. AI is designed from a set of algorithms that are used for the analysis and interpretation of data while it also utilizes symbolic programming to deal with problem-solving. The three analytical skills that AI programming focuses on are:[2]
1. Learning
Gathering data and formulating rules for turning the data into useful information
2. Reasoning
Selecting the best algorithm to achieve the desired outcome
3. Self-Correction
Fine-tuning algorithms on a regular basis for a guaranteed production of the most accurate results feasible
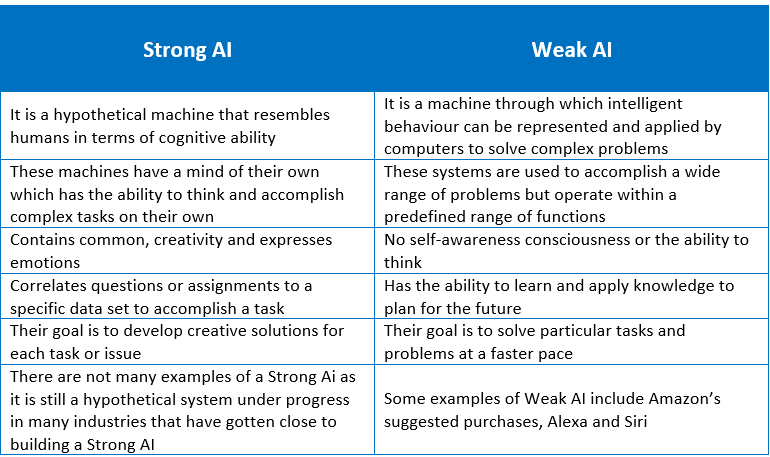
Furthermore, AI can also be classified into two main types which are Weak/Narrow AI or Strong AI. Weak AI or Narrow AI are machines that are designed to give responses or answers that were pre-determined. On the other hand, a Strong AI is a machine that almost mimics a human being by performing tasks on its own with no pre-designed or pre-determined responses incorporated within. The table below depicts the differences between a strong and a weak AI.[3]
Artificial Intelligence Related to Pharmaceutical Industries
AI is a rapidly developing technology that has found many applications in multiple aspects of life and industries with recent advancements in its use in the pharmaceutical industry. The pharmaceutical industry has recently discovered new and innovative methods to employ this technology for tackling some of the industry’s most critical issues. AI in the pharmaceutical industry refers to the use of automated algorithms to perform activities that usually require human intelligence. Today AI is composed of more complex algorithms that mimic the activity of a human brain. AI has the ability to improve diagnostic accuracy, efficacy in provider workflow, clinical operations, procedure accuracy, and overall patient outcomes. It also enables better disease and therapeutic monitoring.[4] The timeline of the evolution of AI in the pharmaceutical field depicts the progressive growth and development of AI in a chronological order.

AI Used in Clinical Trial Industry
Today the medical field is believed to have an enhancing dependency on AI-equipped technologies. This dependency has led to the incorporation of AIs in clinical trial studies. These AI-incorporated clinical trial studies are called Decentralized Clinical Trials (DCTs). DCTs are trials centred around patients’ needs that improve the patients’ experience and attempt to ease their participation by reducing or eliminating the need to travel to specific sites. Conventional clinical trials rely profoundly on trained study coordinators, research assistants, nursing staff, and physicians for data collection and compilation. DCTs have eliminated the need for the presence of trained professionals at the site of clinical trials by making these functions virtual and have also allowed subject recruitment, delivery and administration of study medication, and acquisition of trial outcomes data to proceed without in-person contact between the study team and the patient. DCTs utilize virtual tools like telemedicine, sensory-based technologies, wearable medical devices, home visits, and patient-driven virtual health care interfaces with direct delivery of study drugs and materials to participants’ homes.[5] Among the various reasons leading to the enhanced use of DCTs, COVID-19 had a great role to play. It has been quite evident that COVID-19 has greatly changed the way of living and has had its impact on many fields including many clinical trial studies. The article ‘Impact of COVID-19 on clinical trials and clinical research: A systematic review’[6] stated through a survey that the ability to conduct ongoing clinical trials during the COVID-19 pandemic had affected 69% of the respondents, whereas 78% alleged that COVID-19 affected the initiation of new trials. The COVID-19 pandemic disordered many clinical trials that were expected to bring potential therapeutic options to the market and also caused delays in releasing potentially beneficial therapeutics for patients in need. The changed lifestyle and the necessity to maintain a constant social distancing have encouraged researchers to develop AI-incorporated clinical trials or DCTs. Therefore, in a fully decentralized clinical trial, patient recruitment, delivery along with the administration of study medication, and acquisition of trial outcome data can be processed without involving in-person contact between the study team and the patient.
There are various companies that are developing AI-equipped clinical trials with the aim to reduce the trial size and accelerate clinical development. Alpenglow Biosciences has developed an end-to-end 3D spatial biology platform in collaboration with CorePlus. The company has developed this 3D spatial biology platform which enables 3D datasets to identify 3D biomarkers that could be used to improve patient selection for clinical trials.
AI Used in Other Sectors
It has been quite evident that AI has the ability to provide substantial improvement in all areas of healthcare from diagnostics to treatment. In many fields, AI has already replaced the need for humans for various tasks an example of which would be, analysing medical images or correlating symptoms and biomarkers through Electronic Medical Records (EMRs) with the characterization and prognosis of the disease. On the other hand, healthcare institutions also find the need to keep up with the advancement in technologies due to the high expectation of the patients in terms of services and outcomes. The following diagram depicts the use of AI in different sectors related to healthcare:[7][8]

Companies Using AI
Many companies are using the science of AI for developing new and innovative products due to the great increase in consumer demand for AI based products along with the convenience provided by these technologies. The following diagram depicts the various medical sectors along with the companies working in those fields:[9]

Conclusion
Today, almost all the platforms that we use in our day-to-day life are able to detect the activities we do. All these data can be used for personal profiling while adding a great amount to behavioural understanding and targeting. With AI taking over the basic aspects of life, it is right on the path to conquering the pharmaceutical world. AI holds many advantages and disadvantages on its shoulders. As many researchers find it very convenient to add AI in clinical trial procedures, there still are many that are sceptical related to the potential of AI in the pharmaceutical sector. AIs have made it possible for patients to manage health situations at home and when needed, get in touch with a healthcare worker who in turn will refer them to a specialized physician. On the other hand, AIs have also come to help in situations like the COVID-19 pandemic. Therefore, at the time of an outbreak, natural or manmade disaster, or when the patient cannot reach out to the physician physically, AI has become a necessity as a technology that enables remote interactions between patients, physicians, or researchers. Believe it or not but AIs have a great part to play in our lives and are gradually emerging in the pharmaceutical and other sectors.
References:
- http://pharmabiz.com/ArticleDetails.aspx?aid=141925&sid=9
- https://www.mygreatlearning.com/blog/what-is-artificial-intelligence/
- https://chethankumargn.medium.com/artificial-intelligence-definition-types-examples-technologies-962ea75c7b9b
- https://www.giejournal.org/article/S0016-5107(20)34466-7/fulltext
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8093545/pdf/main.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7538012/#:~:text=The%20survey%20results%20showed%20that,the%20initiation%20of%20new%20trials
- https://www.mdinetworx.com/artificial-intelligence-use-to-improve-claims-processing-for-payer-provider
- https://www.slideshare.net/KaustavDey21/artificial-intelligence-in-field-of-pharmacy
- http://analytics.dkv.global/data/pdf/AIDrugDiscoveryLandscapeOverview2017.pdf
- https://www.chg-meridian.co.uk/resource-centre/blog/advantages-and-disadvantages-of-artificial-intelligence-in-healthcare.html
- https://sasvba.com/blogs/advantages-and-disadvantages-of-artificial-intelligence/