Insights+: EMA Marketing Authorization of New Drugs in August 2024
Shots:
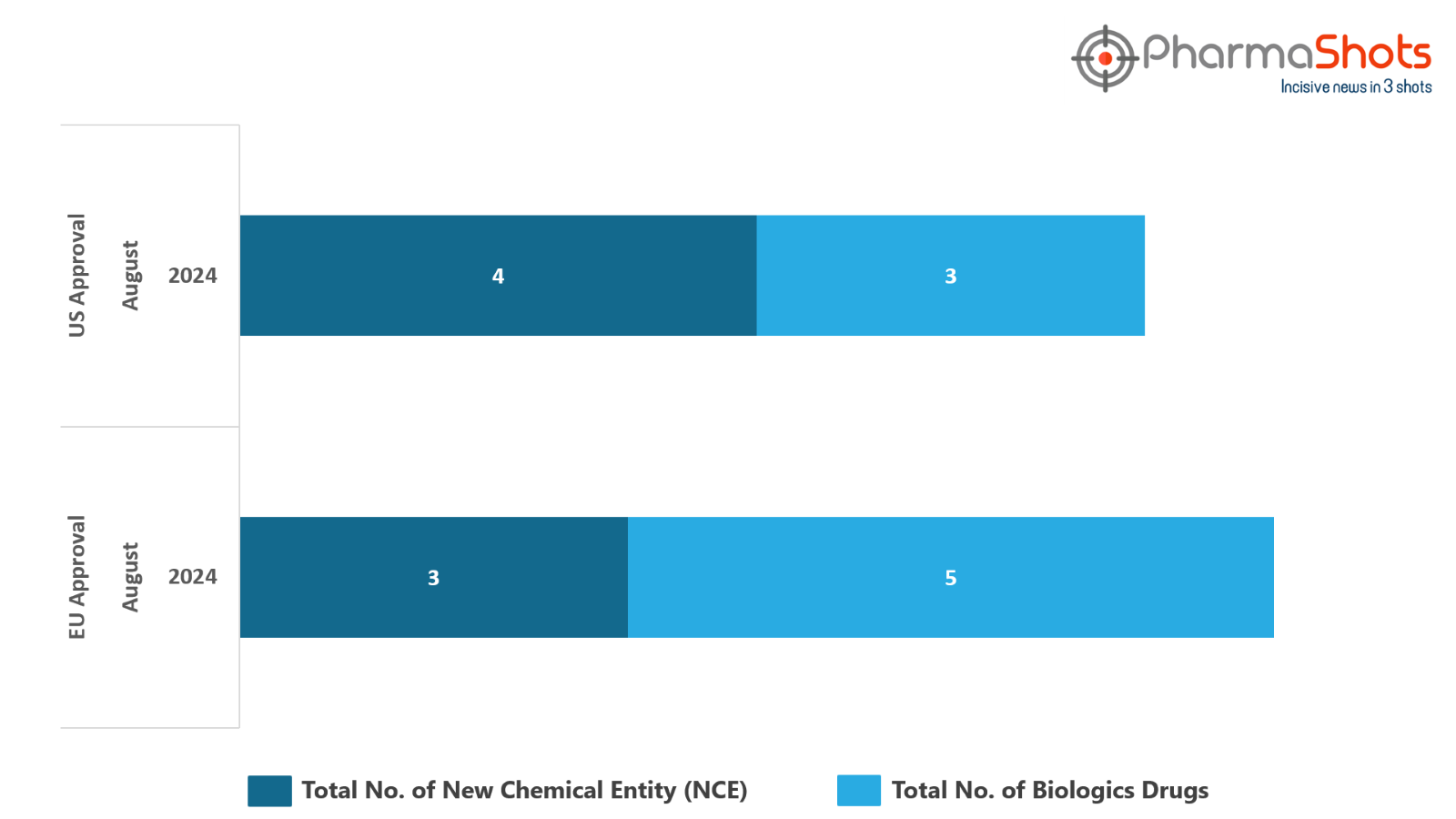
- The EC has approved to 5 Biologics and 3 New Chemical Entities in August 2024, leading to treatments for patients and advances in the healthcare industry
- The major highlighted drugs were Johnson & Johnson’s Balversa to treat Metastatic Urothelial Carcinoma (mUC) and Takeda’s Adzynma for Congenital Thrombotic Thrombocytopenic Purpura (cTTP)
- PharmaShots has compiled a list of 8 drugs that have been approved by the EC
Product Name: Adzynma
Active ingredient: Recombinant ADAMTS13
Company: Takeda
Date: Aug 01, 2024
Disease: Congenital Thrombotic Thrombocytopenic Purpura (cTTP)
Shots:
- The EC has approved Adzynma (recombinant ADAMTS13) to treat ADAMTS13 deficiency in cTTP adults & children. Adzynma is also being assessed under P-IIb study for immune-mediated thrombotic thrombocytopenic purpura (iTTP)
- Approval was based on the P-III study assessing Adzynma to treat cTTP, published in the NEJM, with patients receiving Adzynma (40IU/kg, IV, weekly) or plasma-based therapy for mos.1-6 (period 1), switched treatments for mos.7-12 (period 2) & then Adzynma for mos.13-18 (period 3)
- Study depicted 0 (Adzynma) vs 1 (plasma-based therapy) acute TTP event and 1 vs 7 (in 6 patients) subacute TTP events in periods 1 & 2. Efficacy data from period 3 was consistent with periods 1 & 2
Product Name: mRESVIA
Active ingredient: mRNA-1345
Company: Moderna
Date: Aug 22, 2024
Disease: Lower Respiratory Tract Disease (LTRD)
Shots:
- The EC’s approval of mRESVIA (mRNA-1345) vaccine to prevent LTRD due to RSV infection among adults was supported by P-III (ConquerRSV) trial in adults (n=37,000; ≥60yrs.) & is valid across EU plus as Iceland, Liechtenstein & Norway
- Primary analysis (3.7mos. median follow-up) depicted vaccine efficacy (VE) of 83.7%, published in the NEJM. Supplementary analysis (8.6mos. median follow-up) showed sustained VE of 63.3% against RSV-LRTD incl. ≥2 symptoms with VE of 74.6% & 63% with ≥2 & ≥3 symptoms, respectively
- In addition, the vaccine received the US FDA’s approval in May 2024 for the same. The company has filed MAA to other global authorities
Product Name: Eurneffy
Active ingredient: Epinephrine
Company: ARS Pharmaceuticals
Date: Aug 22, 2024
Disease: Type I Allergic Reactions (Anaphylaxis)
Shots:
- Following the US FDA approval, the EC has approved Eurneffy (2mg) for type I allergic reactions (anaphylaxis) in adults & children (≥30kg). It will be available in Q4’24 through a pharmaceutical company
- The approval was supported by the results from a study, involving ~1200 administrations among >700 subjects, along with studies and peer-reviewed literature supporting these results
- The study assessed PK/PD of Eureffy (2mg) under various conditions such as single and repeat dosing, self-administration, pediatric dosing & in nasal conditions like congestion and rhinorrhea due to allergens or infections like cold/flu
4. Roche’s PiaSky Receives the EC’s Approval to Treat Paroxysmal Nocturnal Haemoglobinuria (PNH)
Product Name: PiaSky
Active ingredient: Crovalimab
Company: Roche
Date: Aug 22, 2024
Disease: Paroxysmal Nocturnal Hemoglobinuria (PNH)
Shots:
- The EC has granted approval to PiaSky (crovalimab) for treating PNH in adults & adolescents (≥12yrs., weight: ≥40kg) who are either treatment-experienced or treatment-naïve
- Approval was based on P-III (COMMODORE 2) trial assessing PiaSky vs eculizumab in PNH patients not treated with C5 inhibitors plus results from another P-III trials, COMMODORE 1 (PNH patients switching from C5 inhibitors) & COMMODORE 3 (new to C5 inhibitor treatment in China)
- COMMODORE 2 showed PiaSky (SC, Q4W) controlled disease, was well-tolerated & non-inferior to eculizumab (C5 inhibitor, given IV, Q2W) with similar safety profiles & AE rates
5. Johnson & Johnson’s Balversa (Erdafitinib) Receives the EC’s Approval to Treat Urothelial Carcinoma
Product Name: Balversa
Active ingredient: Erdafitinib
Company: Johnson & Johnson
Date: Aug 22, 2024
Disease: Metastatic Urothelial Carcinoma (mUC)
Shots:
- The EC has approved Balversa (oral, QD) monotx. for treatment-experienced adults with inoperable or metastatic urothelial carcinoma (mUC) having susceptible FGFR3 genetic alterations
- Approval was supported by results of cohort 1 from the P-III (THOR) trial assessing the safety & effectiveness of Balversa (n=136) vs CT (n=130) to treat mUC with select FGFR alterations and has progressed post previous treatments
- In Jun 2023, the THOR study was stopped early due to positive interim results, allowing CT patients to switch to Balversa. Erdafitinib showed mOS of 12.1mos. vs 7.8mos. & mPFS of 5.6mos vs 2.7mos., with a confirmed ORR of 35.3% vs 8.5%
Product Name: Winrevair
Active ingredient: Sotatercept
Company: Merck
Date: Aug 26, 2024
Disease: Pulmonary Arterial Hypertension (PAH)
Shots:
- The EC has granted approval to Winrevair (45 & 60mg) combined with other PAH therapies to treat PAH, valid across whole EU as well as Iceland, Liechtenstein & Norway
- Approval was based on the P-III (STELLAR) study evaluating the safety & efficacy of Winrevair (target dose 0.7mg/kg, SC, Q3W; n=163) vs PBO (n=160) + stable background therapy in PAH patients (N=323)
- Winrevair improved the 1EP of 6-minute walk distance of 40.8 meters at 24wks. as well as 2EPs of reducing the death risk from any cause & PAH clinical worsening by 82% (number of events: 7 vs 29)
Product Name: Ordspono
Active ingredient: Odronextamab
Company: Regeneron
Date: Aug 26, 2024
Disease: Follicular Lymphoma & Diffuse Large B-cell Lymphoma (FL & DLBCL)
Shots:
- The EC has approved Ordspono for treating r/r FL or r/r DLBCL in patients who have progressed after ≥2L of systemic therapy
- Approval was supported by Ordspono’s P-I (ELM-1; n=60) trial in patients with CD20+ B-cell malignancies, incl. those who progressed post CAR-T therapy & P-II (ELM-2; n=128) trial for 5 B-cell lymphoma subtypes such as DLBCL, FL, mantle cell lymphoma, marginal zone lymphoma
- R/R FL patients (ELM-2) had an ORR of 80% & CR in 73% (mDoR: 25mos.); r/r DLBCL patients naïve to CAR T therapy (ELM-2) had an ORR of 52% & CR in 31% (mDoR: 18mos.); r/r DLBCL patients who progressed post CAR-T therapy (ELM-1) had an ORR of 48% & CR in 32% (mDoR: 15mos.
Product Name: Akantior
Active ingredient: Polihexanide
Company: SIFI and Avanzanite Bioscience
Date: Aug 27, 2024
Disease: Acanthamoeba Keratitis (AK)
Shots:
- Following recommendations from the EMA’s CHMP & COMP, the EC has approved Akantior to treat AK among adults & adolescents (≥12yrs.)
- Approval was based on the data from P-III (ODAK) study in AK patients (n=135), published in Ophthalmology, demonstrating that the disease cured in 84.8%, full vision restoration was found in 66.7% with none of them requiring optical cornea transplant and 7.5% needed a therapeutic cornea transplant
- Avanzanite received the exclusive commercialization rights of Akantior in European Economic Area and Switzerland as per an agreement b/w the company and SIFI
Related Post: Insights+: EMA Marketing Authorization of New Drugs in July 2024





