Insights+: EMA Marketing Authorization of New Drugs in December 2023
Shots:
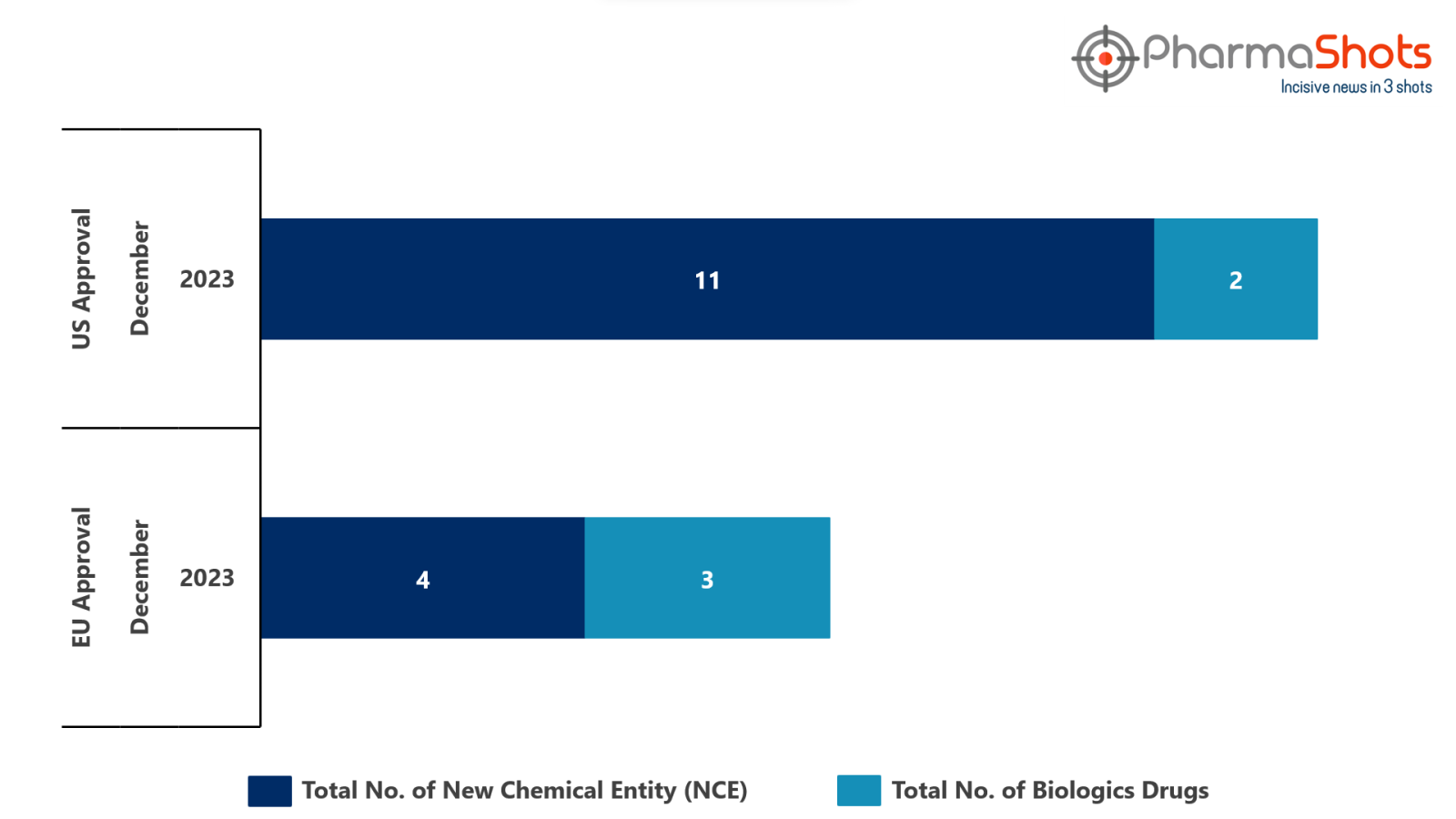
- The EMA approved 3 BLA while 4 New Chemical Entities in December 2023, leading to treatments for patients and advances in the healthcare industry
- In December 2023, the major highlighted drugs were Jempreli to treat dMMR/MSI-H Primary Advanced or Recurrent Endometrial Cancer and Zilbrysq for the treatment of Generalized Myasthenia Gravis
- PharmaShots has compiled a list of a total of 7 new drugs approved by the EMA in December 2023
Product Name: Jempreli
Active ingredient: Dostarlimab
Company: GSK
Date: Dec 11, 2023
Disease: Endometrial Cancer
- The EC has approved Jemperli + carboplatin-paclitaxel to treat dMMR/MSI-H primary advanced/recurrent endometrial cancer. This also converts its previous conditional approval into full approval for the same post-progression on a Pt-containing regimen
- The approval was based on part 1 of the P-III (RUBY) study assessing dostarlimab + carboplatin-paclitaxel followed by dostarlimab vs PBO. Part 2 assesses dostarlimab + carboplatin-paclitaxel followed by dostarlimab + niraparib vs PBO for primary advanced/recurrent endometrial cancer
- Results with a median follow-up of ≥25mos. showed a PFS of 72% with Jemperli added to CT. In a prespecified exploratory analysis for OS in the dMMR/MSI-H population, Jemperli with CT resulted in a 70% reduction in the risk of death vs CT
Product Name: Zilbrysq
Active ingredient: Zilucoplan
Company: UCB
Date: Dec 04, 2023
Disease: Generalized Myasthenia Gravis
- The EC has granted approval to Zilbrysq as an add-on to standard therapy for treating anti-AChR antibody+ gMG, valid across all EU member states along with Iceland, Liechtenstein, and Norway. The drug availability will begin in Q1’24
- The approval was based results from the P-III (RAISE) trial evaluating the efficacy, safety and tolerability of Zilbrysq (0.3mg/kg, SC, 12wks.) vs PBO in adult patients, randomized 1:1, with anti-acetylcholine receptor (AChR) antibody-positive gMG
- The results revealed that at wk12, Zilbrysq showed rapid, consistent and significant improvements in various patient- and clinician-reported outcomes among mild-to-severe anti-AChR antibody+ gMG adult patients
3. Santhera’s Agamree (vamorolone) Receives EC’s Approval to Treat Duchenne Muscular Dystrophy
Product Name: Agamree
Active ingredient: Vamorolone
Company: Santhera
Date: Dec 18, 2023
Disease: Duchenne Muscular Dystrophy
- Followed by the positive opinion from the CHMP, the EC has granted approval to the Santhera’s Agamree (dosing b/w 2 and 6mg/kg/day, for total of 30mos.) for the treatment of DMD patients (aged 4yrs. & +) based on results from the (VISION-DMD) and 3 other trials
- The results showed that Agamree neither reduced bone metabolism nor bone mineralization in the spine after 48wks. and recovered growth & bone health after switching from prednisone. Further data is being collected for its efficacy and safety
- Additionally, Catalyst Pharmaceuticals holds an exclusive license for Agarmee in North America & expects the US launch in Q1’24. Santhera anticipates the drug’s launch in Germany during Q1’24
Product Name: Ayvakyt
Active ingredient: Avapritinib
Company: Blueprint Medicines
Date: Dec 12, 2023
Disease: Indolent Systemic Mastocytosis
- Blueprint Medicines has received approval from the EC for Ayvakyt (avapritinib) to treat adult patients with indolent systemic mastocytosis (ISM)
- Following a positive opinion from the CHMP, the EC approved Ayvakyt based on data from the study (PIONEER) which demonstrated significant improvements compared to PBO in 1EP & 2EP, addressing overall symptoms & mast cell burden along with favorable safety profile
- Ayvakyt, a kinase inhibitor designed to potently and selectively target KIT D816V that is already approved by EC for ISM, adults with ASM, SM-AHN/MCL/GIST
Product Name: lazertinib + Rybrevant
Active ingredient: amivantamab-vmjw
Company: Janssen
Date: Dec 21, 2023
Disease: Lung Cancer
- The submission was based on the P-III (MARIPOSA) clinical trial evaluating lazertinib + Rybrevant vs osimertinib & vs lazertinib alone as a 1L treatment of patients (n=1,074) with locally advanced or metastatic NSCLC with EGFR ex19del or L858R substitution mutations. The 1EP of the study includes PFS & the 2EPs include OS, ORR, DoR, PFS2 & intracranial PFS
- As per the results of the trial, the study depicted a statistically significant & clinically meaningful improvement in PFS for lazertinib + Rybrevant vs osimertinib (23.7 vs 16.6mos.). Janssen presented these results at ESMO 2023
- Lazertinib is an oral brain-penetrant EGFR TKI that targets both the T790M mutation and activating EGFR mutations while sparing wild-type EGFR
6. Cidara Therapeutics Receives European Approval for Rezzayo to Treat Invasive Candidiasis
Product Name: Rezzayo
Active ingredient: Rezafungin
Company: Cidara Therapeutics
Date: Dec 22, 2023
Disease: Invasive Candidiasis
- The approval was led by the positive CHMP opinion & was based on the results from the P-III (ReSTORE) clinical trial evaluating the safety & efficacy of Rezzayo vs SoC (caspofungin) in patients with invasive candidiasis
- The results from the trial depicted a statistical non-inferiority for Rezzayo (QW) vs Soc (BID). These results were supported by the results from the P-II (STRIVE) clinical trial & an extensive nonclinical development program
- Rezzayo, an echinocandin, has received an ODD by the EMA & has been approved by the US FDA. Moreover, as part of its Sep 2019 collaboration with Mundipharma, Cidara will receive a milestone payment of ~$11.14M on Rezzayo’s EU approval
Product Name: Evkeeza
Active ingredient: Evinacumab
Company: Ultragenyx
Date: Dec 18, 2023
Disease: Homozygous Familial Hypercholesterolemia
- The extended approval came following the CHMP’s positive recommendation for Evkeeza in Nov 2023 & was based on the P-III safety, tolerability, PK & efficacy evaluation of Evkeeza (15mg/kg, Q4W) in children aged 5-11yrs. with HoFH. The 1EP of the study includes the change in LDL-C at 24wks. & 2EPs include effect on other lipid parameters
- As per the results, children depicted a reduction in their LDL-C levels by 48% along with a significant reduction in other lipid parameters incl. levels of ApoB, non-HDL-C & total cholesterol
- Evkeeza, an ANGPTL3 inhibitor, received the initial approval by the EC as an adjunct to diet & other lipid-lowering therapies in adolescents & adults aged ≥12yrs. with HoFH in Jun 2021
*The report has been compiled as per available data
Related Post: Insights+: EMA Marketing Authorization of New Drugs in November 2023





