
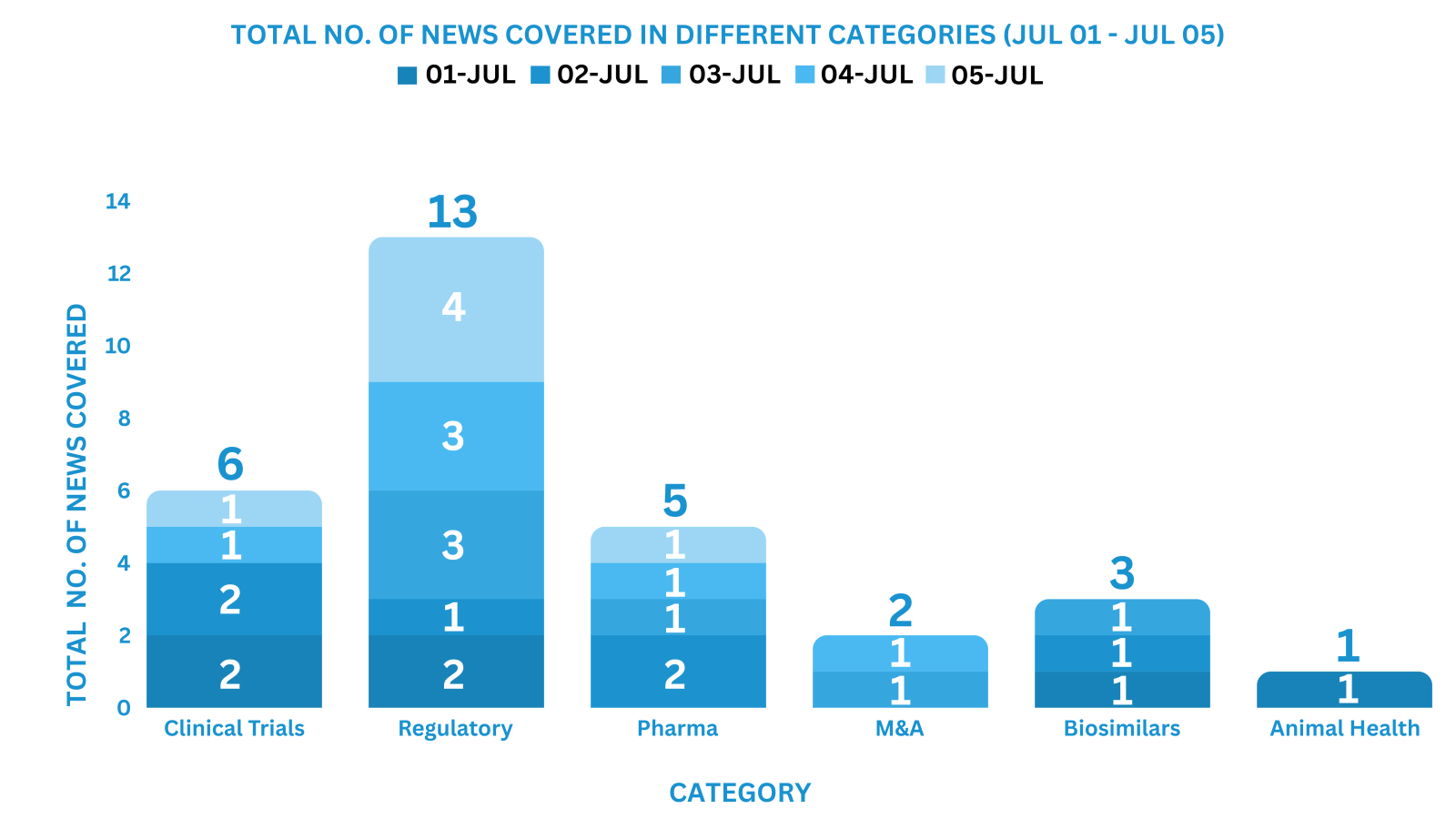
PharmaShots Weekly Snapshots (July 01 – July 05, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Pharma, Biotech, COVID-19, Regulatory & MedTech. Check out our full report below:

Sanofi Reports the P-II Study Data of Frexalimab for the Treatment of Multiple Sclerosis
Read More: Sanofi
AstraZeneca Reports the EMA’s Acceptance of MAA for Sipavibart Under Accelerated Assessment to Prevent COVID-19
Read More: AstraZeneca
Ipsen Reports Collaboration Expansion with Exelixis to Develop Cabometyx for Treating Neuroendocrine Tumors
Read More: Ipsen & Exelixis
HOOKIPA Reports the First Patient Dosing in P-Ib Study of HB-500 to Treat HIV
Read More: HOOKIPA
Roche Reports P-II/III (SKYSCRAPER-06) Trial Data of Tiragolumab to Treat Non-Squamous Non-Small Cell Lung Cancer
Read More: Roche
Zhejiang Doer Biologics Concludes Patient Recruitment in P-Ib/IIa Study of DR10624 Among Obese Individuals
Read More: Zhejiang Doer Biologics

Roche Reports the CHMP’s Positive Opinion of Vabysmo for Retinal Vein Occlusion (RVO)
Read More: Roche
AbbVie’s Tepkinly (Epcoritamab) Gains the CHMP’s Positive Opinion to Treat R/R Follicular Lymphoma
Read More: AbbVie
AstraZeneca’s Lynparza Plus Imfinzi Receives the CHMP’s Positive Opinion for Treating Endometrial Cancer
Read More: AstraZeneca
Eli Lilly Reports the US FDA’s Approval of Kisunla (donanemab-azbt) to Treat Early Symptomatic Alzheimer's Disease
Read More: Eli Lilly
Regeneron and Sanofi Report the EC’s Approval of Dupixent to Treat Chronic Obstructive Pulmonary Disease (COPD)
Read More: Regeneron & Sanofi
Johnson & Johnson’s Sirturo Gains the US FDA & EC’s Approval to Treat Pulmonary Tuberculosis
Read More: Johnson & Johnson
HUTCHMED Reports the NMPA’s NDA Acceptance of Tazemetostat for Treating Follicular Lymphoma
Read More: HUTCHMED
Full-Life Technologies’ 225Ac-FL-020 Gains the US FDA’s Fast Track Designation to Treat Metastatic Castration-Resistant Prostate Cancer
Read More: Full-Life Technologies
Medigene Receives Chinese Patent for MDG1015 to Treat Various Solid Tumors
Read More: Medigene
Roche’s Vabysmo Prefilled Syringe Receives the US FDA’s Approval for Vision Loss
Read More: Roche
AstraZeneca’s Tagrisso Plus Chemotherapy Receives the EC’s Approval to Treat EGFR-Mutated Advanced Lung Cancer
Read More: AstraZeneca
Eleva’s CPV-104 Gains EC’s Orphan Drug Designation to Treat C3 Glomerulopathy (C3G)
Read More: Eleva
Biostar Pharma Reports the US FDA’s IND Clearance for P-II Trial of Utidelone to Treat Breast Cancer Brain Metastasis
Read More: Biostar Pharma

Ubix Therapeutics Collaborates with Yuhan to Develop UBX-103 for Treating Metastatic Castration Resistant Prostate Cancer
Read More: Ubix Therapeutics & Yuhan
Radionetics Oncology and Eli Lilly Collaborate to Develop Small Molecule Radiopharmaceuticals
Read More: Radionetics & Eli Lilly
GSK and CureVac Report Restructuring of their Partnership
Read More: GSK & CureVac
Ascentage Pharma Obtains an Option Payment of $100M from Takeda
Read More: Ascentage & Takeda
Pendopharm Collaborates with Ascendis Pharma to Commercialize TransCon PTH for Treating Hypoparathyroidism in Canada
Read More: Pendopharm & Ascendis Pharma

Samsung Bioepis Reports the US FDA’s Approval of Pyzchiva (Biosimilar, Stelara)
Read More: Samsung Bioepis
Kashiv BioSciences Partners with Amneal Pharmaceuticals to Commercialize ADL018 (Biosimilar, Xolair)
Read More: Kashiv BioSciences & Amneal Pharmaceuticals
Alvotech Reports Data from Confirmatory Trial of AVT03 (Biosimilar, Prolia and Xgeva)
Read More: Alvotech
Formycon and Klinge Biopharma Reports the US FDA’s Approval of Ahzantive (Biosimilar, Eylea)
Read More: Formycon & Klinge Biopharma
Tanvex BioPharma Reports the US FDA’s Approval of Nypozi (Biosimilar, Neupogen)
Read More: Tanvex

Can-Fite Reports Data from its Partner Vetbiolix’s Clinical Study of Piclidenoson in Dogs with Osteoarthritis
Read More: Can-Fite

Pentixapharm Reports the Acquisition of Glycotope’s Target Discovery Business
Read More: Pentixapharm & Glycotope
Quest Diagnostics Reports the Acquisition of LifeLabs from OMERS for an Aggregate of ~$985M
Read More: Quest Diagnostics
Related Post: PharmaShots Weekly Snapshots (June 24 – June 28, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














