
PharmaShots Weekly Snapshots (June 24 – June 28, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Pharma, Biotech, COVID-19, Regulatory & MedTech. Check out our full report below:
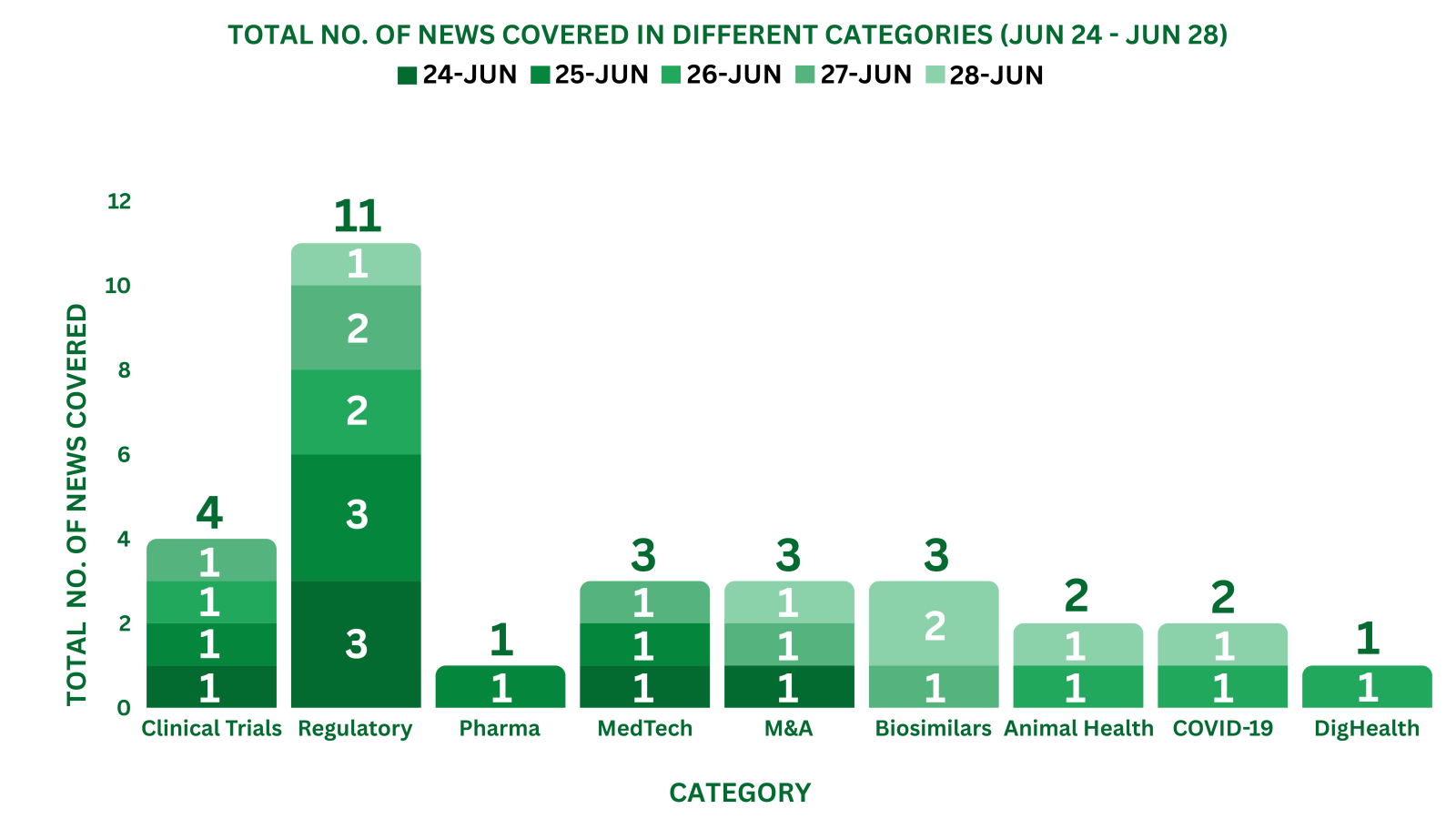

Eli Lilly Reports Detailed Data from the P-III (SURMOUNT-OSA) Trial of Tirzepatide to Treat Obstructive Sleep Apnea
Read More: Eli Lilly
AstraZeneca Reports Updated Data from the P-III (ADJUVANT BR.31) Study of Imfinzi to Treat Non-Small Cell Lung Cancer
Read More: AstraZeneca
Sanofi Reports the P-II Study Data of Riliprubart to Treat Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Read More: Sanofi
Neurobo Pharmaceuticals Reports First Patient Dosing in P-I Study of DA-1726 to Treat Obesity
Read More: Neurobo Pharmaceuticals

Takeda’s Fruzaqla Gains the EC’s Approval for the treatment of Metastatic Colorectal Cancer
Read More: Takeda
BMS’ Krazati Receives the US FDA’s Accelerated Approval to Treat Locally Advanced or Metastatic Colorectal Cancer (mCRC)
Read More: BMS
argenx’ Vyvgart Hytrulo Bags the US FDA’s Approval for Treating Chronic Inflammatory Demyelinating Polyneuropathy
Read More: argenx
GSK Reports the Japanese MHLW’s Approval of Omjjara (momelotinib) for Treating Myelofibrosis
Read More: GSK
Daiichi Sankyo Reports the MHLW’s Approval of Ezharmia (Valemetostat Tosilate) To Treat Peripheral T-Cell Lymphoma
Read More: Daiichi Sankyo
Roche’s Ocrevus SC (Ocrelizumab) Receives the EC’s Approval to Treat Multiple Sclerosis
Read More: Roche
AstraZeneca’s Tagrisso Plus Chemotherapy Receives Japanese Approval to Treat EGFR-Mutated Advanced Lung Cancer
Read More: AstraZeneca
AbbVie Receives Complete Response Letter for ABBV-951's NDA to Treat Parkinson's Disease
Read More: AbbVie
AstraZeneca’s Tagrisso Plus Chemotherapy Receives NMPA’s Approval to Treat EGFR-Mutated Advanced Lung Cancer
Read More: AstraZeneca
AbbVie’s Epkinly (epcoritamab-bysp) Receives the US FDA’s Accelerated Approval to Treat R/R Follicular Lymphoma
Read More: AbbVie
Tubulis Reports the US FDA’s Fast Track Designation of TUB-040 to Treat Ovarian Cancer
Read More: Tubulis
Roche’s PiaSky Receives the CHMP’s Positive Opinion for TreatingParoxysmal Nocturnal Haemoglobinuria (PNH)
Read More: Roche

Altaris Reports the Acquisition of Sharecare
Read More: Altaris & Sharecare
Quest Diagnostics Reports the Acquisition of Lab Assets from Allina Health
Read More: Quest Diagnostics & Allina Health
AbbVie Reports the Acquisition of Celsius Therapeutics
Read More: AbbVie & Celsius Therapeutics

The US FDA Grants Clearance to Alcon’s Unity VCS and Unity CS for Eye Care
Read More: Alcon
Evolus Reports Premarket Approval Application Submission to the US FDA for Evolysse Dermal Filler Products
Read More: Evolus
Tempus Reports the US FDA’s Approval of Tempus ECG-AF to Detect Patients at Risk of Atrial Fibrillation
Read More: Tempus

Rani Therapeutics Collaborates with ProGen to Develop RT-114 for Obesity
Read More: Rani Therapeutics & ProGen

Merck Animal Health’s Nobivac NXT Canine Flu H3N2 Receives the USDA’s Approval for Canine Influenza
Read More: Merck Animal Health
PetPace Brings Out AI-Based Pregnancy Monitoring Module to Improve Pet Health at EVSSAR 2024
Read More: PetPace

The EC Approves Biogen’s Tofidence (Biosimilar, Roactemra) for Treating Arthritis and COVID-19
Read More: Biogen
Pfizer and BioNTech Report the CHMP’s Positive Opinion of Comirnaty JN.1 Vaccine for COVID-19
Read More: Pfizer & BioNTech

InfoBionic.Ai Collaborates with Anumana to Advance AI-Based Technology for Early Detection of Cardiac Diseases
Read More: InfoBionic.Ai & Anumana

The NMPA Approves Simcere Zaiming’s Enlituo (Biosimilar, Cetuximab Beta) as a 1L Treatment for Metastatic Colorectal Cancer (mCRC)
Read More: Simcere Zaiming
Coherus Reports the Divestment of Yusimry (Biosimilar, Humira) to Hong Kong King-Friend Industry
Read More: Coherus
Related Post:- PharmaShots Weekly Snapshots (June 17 – June 21, 2024)

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














