Insights+: The US FDA New Drug Approvals in April 2023
Shots:
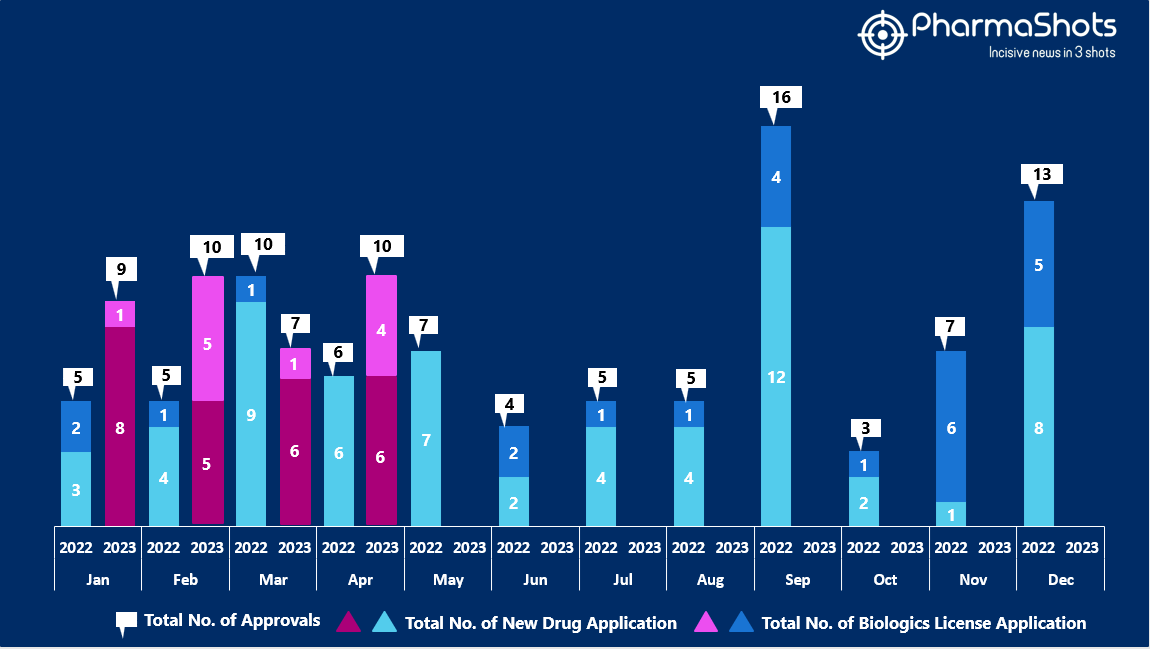
- The US FDA approved 6 NDAs and 4 BLA in April 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 36 novel products in 2023
- In April 2023, the major highlights drugs were, Trikafta approval for children with cystic fibrosis, Qalsody for the treatment of amyotrophic lateral sclerosis
- PharmaShots has compiled a list of a total of 10 new drugs approved by the US FDA in April 2023
Keytruda
Active ingredient: pembrolizumab Approved: April 04, 2023
Company: Merck Disease: Urothelial Cancer
- The US FDA has approved Merck’s Keytruda (anti-PD-1 therapy) in combination with Padcev for adult patients with LA/mUC who are not eligible for cisplatin-containing CT
- The approval was based on the (KEYNOTE-869) open-label, multi-cohort (dose escalation cohort, cohort A, cohort K) study evaluating Keytruda + enfortumab vedotin in 121 patients. The study was conducted in collaboration with Seagen & Astellas
- The results showed ORR of 68% with CR and PR rates of 12% and 55%, respectively in the combined efficacy analysis of the dose escalation cohort, cohort A & cohort K (n=121). For the dose escalation cohort + cohort A & cohort K, m-DoR was 22.1mos. & was not reached, median follow-up time 44.7 & 14.8mos., respectively
IntelGenx’s Rizafilm Receives the US FDA’s Approval for the Treatment of Acute Migraine
Rizafilm
Active ingredient: rizatriptan Approved: April 17, 2023
Company: IntelGenx Disease: Acute Migraine
- The US FDA has approved the NDA of Rizafilm VersaFilm (US market name for Rizaport), an oral thin film formulation of rizatriptan benzoate for the treatment of acute migraine
- The approval was based on results from a bioequivalence study evaluating Rizafilm vs the US reference Maxalt-MTL and the EU reference Maxalt-Lingua in 30 healthy volunteers which showed that Rizaport was bioequivalent to the US reference Maxalt-MTL and the EU reference Maxalt-Lingua
- Under the Dec 2018 agreement, IntelGenx collaborated with Gensco for the exclusive commercialization of Rizafilm in the US. Additionally, Rizafilm is expected to be commercially available in the US shortly
Gamida Cell’s Omisirge (omidubicel-onlv) Receives the US FDA’s Approval for Hematologic Malignancies
Omisirge
Active ingredient: omidubicel Approved: April 18, 2023
Company: Gamida Cell Disease: Hematologic Malignancies
- The US FDA has approved Omisirge to reduce the risk of infection in children & adults aged ≥12yrs. with hematologic malignancies who have a planned umbilical cord blood transplantation after a myeloablative conditioning regimen
- The approval was based on the P-III trial evaluating omidubicel vs transplantation of umbilical cord blood in 125 patients which showed that 87% of patients with Omisirge achieved neutrophil recovery with a median of 12 days vs 83% in the umbilical cord group with a median of 22 days
- 39% vs 60% experienced a bacterial or fungal inf. within 100 days after transplantation. The safety profile was consistent with the expected AEs of Allo-HSCT, following myeloablative conditioning & the full results are available in Blood
Qulipta
Active ingredient: atogepant Approved: April 18, 2023
Company: AbbVie Disease: Chronic Migraine
- The approval was based on the P-III trial (PROGRESS) evaluating Qulipta (60mg, qd) vs PBO in adult patients
- The trial met its 1EPs i.e., a reduction from baseline in mean monthly migraine days across 12wk. treatment period & improvement in all six 2EPs incl. 50% reduction in mean MMDs @12wk. along with improvements in function & reduction in activity impairment due to migraine. The efficacy results were consistent with (ADVANCE) episodic migraine trial & overall safety profile was consistent with the EM patient population
- Qulipta, an oral CGRP receptor antagonist was available in the US for migraine in adults. The product is available in 10/30/60mg strengths for EM while a 60mg dose is indicated for chronic migraine patients
Polivy
Active ingredient: polatuzumab vedotin Approved: April 20, 2023
Company: Genentech Disease: Diffuse Large B-cell Lymphoma
- The US FDA has approved Polivy in combination with Rituxan, cyclophosphamide, doxorubicin & prednisone (R-CHP) for adult patients before untreated DLBCL
- The approval was based on the P-III trial (POLARIX) evaluating Polivy + R-CHP vs R-CHP in a ratio (1:1) in 1000 patients. The trial is being conducted in collaboration with LYSA & LYSARC
- The results showed an improvement in PFS, a 27% reduction in risk of disease progression, relapse, or death. The safety profile was comparable, rates of grade 3-4 AEs (57.7% vs 57.5%), serious AEs (34.0% vs 30.6%), grade 5 AEs (3.0% vs 2.3%) & AEs leading to dose reduction (9.2% vs 13.0%). Polivy + bendamustine & Rituxan are currently approved in 80+ countries globally for r/r DLBCL
Trikafta
Active ingredient: elexacaftor, tezacaftor and ivacaftor Approved: April 26, 2023
Company: Vertex Disease: Diffuse Large B-cell Lymphoma
- The US FDA has approved the expanded use of Trikafta (elexacaftor/tezacaftor/ivacaftor and ivacaftor) in children aged 2-5yrs. with CF with 1 F508del mutation in the CFTR gene or a mutation in the CFTR gene responsive to Trikafta based on in vitro data
- The approval was based on the 24wk. two-part P-III trial evaluating Trikafta which showed that the therapy was well tolerated with a safety profile consistent with that observed in older age groups, improvement in sweat chloride concentration, a measure of CFTR function & lung function. The results were published in the AJRCCM
- The therapy was approved in the US, Canada, Switzerland, Australia, New Zealand & Israel, EU, the UK, Iceland, Liechtenstein & Norway as Kaftrio in combination with Kalydeco
Qalsody
Active ingredient: tofersen Approved: April 26, 2023
Company: Biogen Disease: Amyotrophic Lateral Sclerosis
- The US FDA has granted accelerated approval to Qalsody (100mg/15mL) for adults with ALS who have a mutation in the SOD1 gene. The indication was approved based on a reduction in plasma NfL
- The approval was based on 28wk. P-III study (VALOR) evaluating tofersen vs PBO in a ratio (2:1) in 108 adults aged 23-78yrs. In the primary analysis population, less decline from baseline as measured by ALSFRS-R & the results were not statistically significant
- In the overall ITT population, 55% reduction in plasma NfL vs 12% increase in PBO. Levels of CSF SOD1 protein, an indirect measure of target engagement was reduced by 35% vs 2%. Findings from an interim analysis at 52wks. showed similar reductions in NfL in patients previously receiving PBO who initiated tofersen in OLE & those who received tofersen in 28wk. study
Abilify Asimtufii
Active ingredient: aripiprazole Approved: April 27, 2023
Company: Otsuka Disease: Schizophrenia or Bipolar I Disorder
- The US FDA has approved the NDA for Abilify Asimtufii (IM, q2mos.) extended-release injectable suspension for schizophrenia or maintenance monotx. treatment of bipolar I disorder in adults
- The approval was based on the results from a 32wk. PK bridging analysis evaluating Abilify Asimtufii in 266 patients with schizophrenia & bipolar I disorder across multiple care centers while investigators assessed 960 & 720mg doses across trial participants
- Both doses met 1EPs & demonstrating similar aripiprazole plasma concentrations and comparable efficacy to Abilify Maintena (400mg, qm) treatment dose. Additionally, the product is available in 720mg/2.4mL and 960mg/3.2mL dosage strengths
VOWST
Active ingredient: fecal microbiota spores, live-brpk Approved: April 27, 2023
Company: Seres Therapeutics Disease: C. Difficile Infection
- The US FDA has approved VOWST (formerly called SER-109), an orally administered microbiota-based therapeutic which is expected to be available in June
- The approval was based on the results from the P-III (ECOSPOR III & IV) published in the NEJM & the JAMA Network Open evaluating VOWST vs PBO in 182 & 263 adult patients. The (ECOSPOR III) trial showed a reduction in CDI recurrence @8wks., (~88% vs 60%) were recurrence-free @8wks.; 79% vs 53% at 6mos. post-treatment, no treatment-related SAEs were seen & the frequency of TRAEs was similar b/w treatment arms
- The (ECOSPOR IV) study results contributed to the VOWST safety database & supported product approval. Seres & Nestlé Health Science collaborated in July 2021 to jointly commercialize VOWST in the US & Canada
Uzedy
Active ingredient: risperidone Approved: April 31, 2023
Company: Teva Disease: Schizophrenia
- The US FDA has approved Uzedy (risperidone) extended-release injectable suspension for the treatment of adults with schizophrenia
- The approval was based on the results from the 2 P-III study (RISE) & (SHINE) evaluating the efficacy of risperidone extended-release injectable suspension for SC use in 544 & 336 patients aged 13-65yrs. with schizophrenia. The results showed that the patients treated with Uzedy achieved a ~80% reduction in risk of schizophrenia relapse over PBO
- Uzedy, the first SC, long-acting formulation of risperidone that has been developed using SteadyTeq (copolymer technology) to MedinCell that controls the steady release of risperidone
Related Post: Insights+: The US FDA New Drug Approvals in March 2023






