Bayer at ASCO GU: Neal Shore Speaking about Nubeqa with PharmaShots
Shots:
- At ASCO GU, Bayer presented new data from its Oncology portfolio. The analysis revolved around NUBEQA (darolutamide), which is currently approved in the US in combination with docetaxel for the treatment of mHSPC and nmCRPC in adults
- Neal Shore in conversation with PharmaShots initiated the discussion by familiarizing our audience with the ARASENS P-III study that studied the OS with darolutamide in combination with androgen deprivation therapy (ADT) and docetaxel vs. Placebo
- Neal shares the highlights of the post hoc sensitivity analysis that was presented at ASCO GU
Saurabh: Can we first understand the study design of the ARASENS study (Ph3)? (This includes patient number, demographics, inclusion criteria, etc)
Neal: ARASENS was the first pivotal trial, to show a successful combination of a novel androgen receptor inhibitor (ARi) with ADT and docetaxel, against androgen deprivation therapy (ADT) and docetaxel in overall survival shifting the needle for mHSPC treatment.
ARASENS was a global randomized double-blind, placebo controlled, multi-center study of 1,305 patients, designed to evaluate survival versus docetaxel (the current standard of care at the time) in men with mHSPC. The primary endpoint is overall survival. Secondary endpoints included time to castration-resistant prostate cancer (CRPC), time to pain progression, time to first symptomatic skeletal event (SSE), time to initiation of subsequent anticancer therapy, all evaluated at 12-week intervals, as well as adverse events (AEs) as a measure of safety and tolerability.
Saurabh: What was the aim of post hoc sensitivity analysis and why was it required or done?
Neal: Real-world data is a critical part of the scientific process – treatments don’t stop at the conclusion of a clinical trial and information about how a therapy performs in the real world is a key part of the decision-making process for clinicians. As such, we’re committed to continuing to evaluate the long-term results for patients treated with darolutamide in the real-world setting and this post-hoc analysis is part of that commitment.
The analysis was designed to evaluate the impact of these subsequent therapies (referred to as informative intercurrent events) in patients who had received darolutamide as part of the Phase III ARASENS trial.
As part of this post-hoc analysis, we counted initiation of subsequent chemotherapy as an event in censored patients still alive at the end of follow-up.
Saurabh: Can we talk about the key highlights results being presented at ASCO GU from the sensitivity analysis of the ARASENS study?
Neal: Post-hoc sensitivity analyses from the Phase III ARASENS trial presented at ASCO GU and were consistent with and supportive of the ARASENS primary OS analysis – reinforcing darolutamide plus ADT plus docetaxel as an effective and well tolerated standard of care in patients with mHSPC.
Saurabh: Shed some light on the primary analysis; you mentioned a significant improvement in OS with DARO + ADT + DOC. How long was the follow-up period, and were there notable differences in the survival curves?
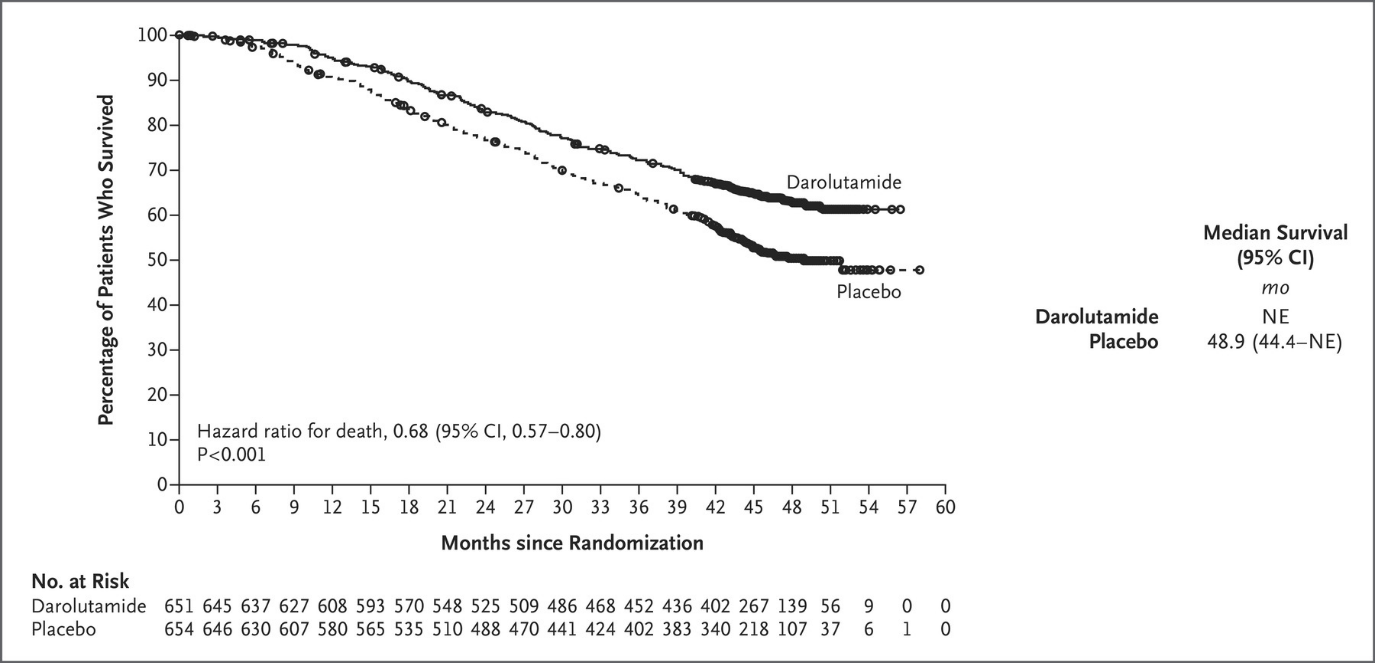
Neal: Results from the ARASENS trial show that darolutamide plus ADT in combination with docetaxel met its primary endpoint of overall survival, reducing the risk of death by 32% (HR=0.68, 95% CI 0.57-0.80; P<0.001), when compared to ADT with docetaxel.
Moreover, the combination also demonstrated improvements across the trial’s secondary endpoints. This included more than doubling the time to progression to castration-resistant prostate cancer (CRPC) at four years versus docetaxel, and a 64% risk reduction in time to CRPC. Additionally, the combination demonstrated a 61% risk reduction in time to initiation of subsequent antineoplastic therapy over docetaxel, delayed time to pain progression and delayed time to first symptomatic skeletal event.
At the data cutoff date for the primary analysis (October 25, 2021), the median treatment duration was longer in the darolutamide group (41.0 months) than in the placebo group (16.7 months), and a higher percentage of patients in the darolutamide group (45.9% [299 of 651 patients]) than in the placebo group (19.1% [125 of 654 patients]) were still receiving the assigned trial treatment. Six cycles of docetaxel were completed in 571 of 652 patients (87.6%) in the darolutamide group and in 556 of 650 patients (85.5%) in the placebo group. The reasons for discontinuation of the trial agent are provided in Figure S1. The median follow-up for overall survival was 43.7 months in the darolutamide group and 42.4 months in the placebo group.
Saurabh: What were the major safety/AEs observed in the sensitivity analysis and overall ARASENS study?
Neal: Safety results in the sensitivity analysis showed similar levels of treatment-emergent adverse events (TEAEs) between groups, with TEAEs leading to darolutamide or placebo discontinuation in 13.5% and 10.5% of patients in each group respectively.
Overall, safety results from ARASENS have confirmed darolutamide plus ADT in combination with docetaxel is well tolerated, with no substantial increase in TEAEs compared to placebo.
Saurabh: How does this sensitivity analysis help DARO overall in mHSPC patients?
Neal: This real-world data confirms the continued benefit of darolutamide plus ADT in combination with docetaxel for mHSPC patients, even when accounting for use of subsequent antineoplastic therapy. Providing key information to support physicians in selecting the right treatment for their patients.
Saurabh: Can we expect a similar analysis to be presented at ASCO later this year from other subtypes of prostate cancer related DARO?
Neal: We continue to collect data and investigate darolutamide across different stages of prostate cancer and will share further data at upcoming scientific meetings.
Image Source: Canva
About the Author:

Neal Shore
US Chief Medical Officer of Urology/ Surgical Oncology, GenesisCare USA Director, Carolina Urologic Research Center
Related Post: Exploring Beyond: Kari Wong Sheds Light on Metabolon’s Strategic Collaboration with SITraN


