
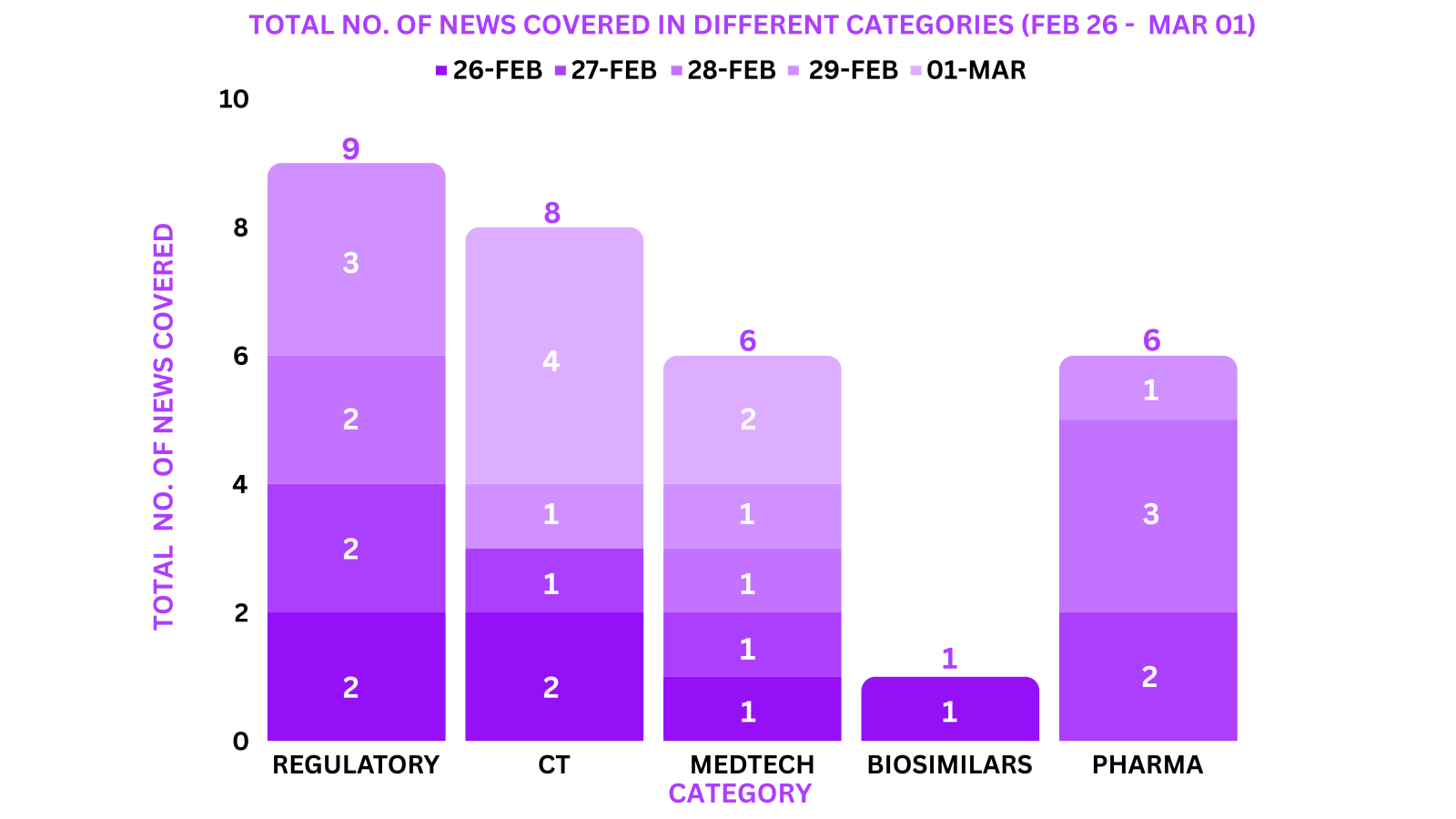
PharmaShots Weekly Snapshots (February 26 – March 01, 2024)
This week PharmaShots’ news was all about the updates on Regulatory, Clinical Trials,Pharma, Biosimilar & MedTech. Check out our full report below:

The CHMP Grants Positive Opinion to BMS’ Reblozyl for the Treatment of Transfusion-Dependent Anemia Due to Myelodysplastic Syndrome (MDS)
Read More: BMS
The CHMP Recommends AstraZeneca’s Voydeya for Approval in the EU to Treat Paroxysmal Nocturnal Haemoglobinuria (PNH) with Residual Haemolytic Anaemia
Read More: AstraZeneca
CHMP Grants Positive Opinion to BeiGene’s Tevimbra (tislelizumab) as a Treatment for Non-Smal Cell Lung Cancer (NSCLC)
Read More: BeiGene
The US FDA Grants the Breakthrough Designation to Bayer’s BAY2927088 for the Treatment of Non-Small Cell Lung Cancer (NSCLC)
Read More: Bayer
The US FDA Accepts Incyte’s BLA for Axatilimab with Priority Review to Treat Chronic Graft-Versus-Host Disease
Read More: Incyte
ARTHEx Biotech Reports the US FDA's IND Clearance to Commence the P-I-IIa (ArthemiR) Study of ATX-01 for Treating Myotonic Dystrophy Type 1 (DM1)
Read More: ARTHEx Biotech
The US FDA Clears Spero Therapeutics’ IND Application of SPR206 for the Treatment of MDR Gram-Negative Bacterial Infections
Read More: Spero Therapeutics
The US FDA Accepts and Grants Priority Review to Applied Therapeutics’ NDA for Govorestat as a Treatment for Classic Galactosemia
Read More: Applied Therapeutics
The US FDA Grants ODD to SN BioScience’s SNB-101 for Treating Pancreatic Cancer
Read More: SN BioScience
Sanofi’s P-II Study of Rilzabrutinib Reveals Reduction in Itch Severity & Improved Efficacy in Adults with Chronic Spontaneous Urticaria (CSU)
Read More: Sanofi
GSK Reports the P-III (EAGLE-1) Trial Results of Gepotidacin for Treating Urogenital Gonorrhoea (GC)
Read More: GSK
Boehringer Ingelheim’s Survodutide, Under P-II Development, Shows Improvement in Metabolic Dysfunction-Associated Steatohepatitis (MASH)
Read More: Boehringer Ingelheim
BioLineRx Doses First Patient with Motixafortide in P-II Study for Treating Pancreatic Ductal Adenocarcinoma (PDAC)
Read More: BioLineRx
Ironwood Pharmaceuticals Highlights P-III Results for Apraglutide to Treat Short Bowel Syndrome with Intestinal Failure (SBS-IF)
Read More: Ironwood Pharmaceuticals
Innovent Reports Updates for IBI3002 Under P-I Clinical Evaluation as a Treatment of Asthma
Read More: Innovent
BeiGene Highlights the Results from a Comparative Analysis of Brukinsa with Acalabrutinib to Treat Chronic Lymphocytic Leukaemia (CLL)
Read More: BeiGene
Cardiff Oncology Highlights the P-II Results for Onvansertib to Treat Colorectal Cancer
Read More: Cardiff Oncology

Virtual Incision’s MIRA Surgical System Gains the US FDA’s Marketing Authorization for Adults Undergoing Colectomy Procedures
Read More: Virtual Incision
The US FDA Grants 510(k) Clearance to Selux Diagnostics’ PBC Separator with Selux AST System for Antibiotic Susceptibility Testing
Read More: Selux Diagnostics
Amber Therapeutics Highlights Data from the Study of Amber-UI Pudendal Neuromodulation System for Urinary Incontinence
Read More: Amber Therapeutics
The US FDA Grants Breakthrough Device Designation to ONWARD Medical’s ARC Brain-Computer Interface (BCI) System
Read More: ONWARD Medical
The US FDA Grants a 51099(k) Clearance to Embolx’s Soldier High-Flow Microcatheter
Read More: Embolx
The MHLW Approves Chugai’s FoundationOne CDx Cancer Genomic Profile as a Companion Diagnostic for the Detection of Solid Tumor
Read More: Chugai

The US FDA Approves Alvotech and Teva Pharmaceutical’s Simlandi, Biosimilar to Humira
Read More: Alvotech & Teva Pharmaceutical
Neomorph and Novo Nordisk Join Forces to Discover Molecular Glue Degraders for Cardiometabolic and Rare Diseases
Read More: Neomorph & Novo Nordisk
Biotheus and Bitterroot Bio have Signed a Multi-Year Research Collaboration Agreement to Develop Bispecific Proteins in Cardio-Immunology
Read More: Biotheus & Bitterroot Bio
Viatris and Idorsia Partner to Develop and Commercialize Selatogrel and Cenerimod
Read More: Viatris & Idorsia
AbbVie and OSE Immunotherapeutics Partner to Develop OSE-230 for Treating Chronic Inflammation
Read More: AbbVie & OSE Immunotherapeutics
Amneal Signs an Exclusive Licensing Agreement with Zambon Biotech for IPX203 to Treat Parkinson’s Disease (PD)
Read More: Amneal Pharmaceuticals & Zambon Biotech
Arcutis and Sato Pharmaceutical for the Development and Commercialization of Topical Roflumilast for Dermatological Indications in Japan
Read More: Arcutis & Sato Pharmaceutical
Related Post:- PharmaShots Weekly Snapshots (February 19 – February 23, 2024)
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.














