Insights+: EMA Marketing Authorization of New Drugs in November 2022
Shots:
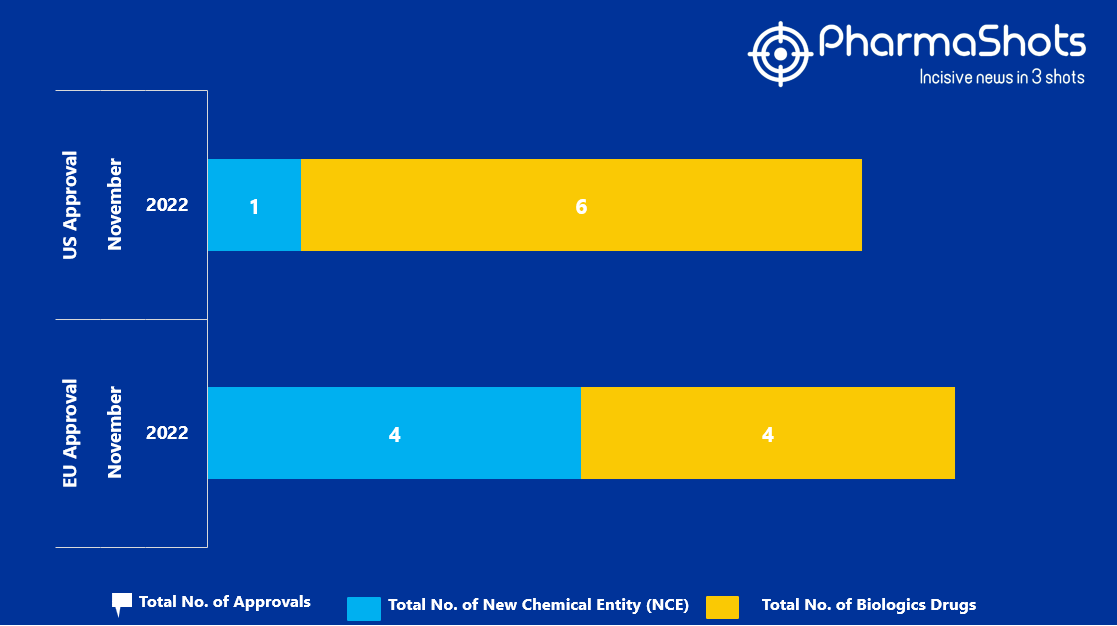
- The EMA approved 4 New Chemical Entity (NCE) and 4 Biologic Drugs in November 2022, leading to treatments for patients and advances in the healthcare industry
- In November 2022, the major highlights drugs were Skyrizi’s approval for active crohn’s disease, Beyfortus for RSV lower respiratory tract disease
- PharmaShots has compiled a list of a total of 8 new drugs approved by the EMA in November 2022
Brukinsa
Active ingredient: zanubrutinib Approved: November 02, 2022
Company: BeiGene Disease: Marginal Zone Lymphoma
- The EC has granted marketing authorization of Brukinsa for r/r MZL who have received one prior anti-CD20-based therapy
- The approval was based on results from the P-II (AGNOLIA) trial which showed a high ORR of 68% with 26% of patients achieving CR as assessed by IRC, responses were observed in all patients regardless of MZL subtypes along with a rapid and durable disease control with a median time to response of 2.8mos., was well-tolerated and safety was consistent with its established profile, low rates of discontinuation due to AEs (3.5%)
- The EC’s approval will apply to all 27 member states of the EU, Iceland, and Norway. Brukinsa has been approved in the EU for adult patients with WM
Beyfortus
Active ingredient: nirsevimab Approved: November 04, 2022
Company: AstraZeneca Disease: RSV Lower Respiratory Tract Disease
- The approval was based on results from the P-III (MELODY), P-II/III (MEDLEY) & P-IIb trials evaluating Beyfortus in patients with RSV lower respiratory tract disease
- In the P-III (MELODY) & P-IIb trial, the therapy met its 1EPs i.e., reduction in the incidence of medically attended LRTI caused by RSV by 74.5% & 70.1% through 151 Days & hospitalization for RSV LRTI through 150 Days Postdose (62.1%) & (78.4%). The therapy also demonstrated a comparable safety & tolerability profile to Synagis in the P-II/III (MEDLEY) trial
- The results from the pre-specified pooled analysis of the P-IIb and (MELODY) trials, 79.5% efficacy was observed against medically attended LRTI while 77.3% against RSV LRTI hospitalizations
Pyrukynd
Active ingredient: mitapivat Approved: November 10, 2022
Company: Agios Disease: Pyruvate Kinase Deficiency
- The marketing authorization was based on the (ACTIVATE) & (ACTIVATE-T) trials evaluating Pyrukynd in adults with PK deficiency
- Both the trial met its 1EPs i.e., In a trial (ACTIVATE), 40% achieved Hb response who are not regularly transfused, & significant improvements were also demonstrated for all pre-specified 2EPs incl. markers of hemolysis & ineffective erythropoiesis
- In the trial (ACTIVATE-T), 37% achieved a transfusion reduction response in the 24wk. fixed dose period over the historical transfusion burden standardized who are regularly transfused, 22% were transfusion-free. The company continues to advance the therapy in P-III (ENERGIZE) & (ENERGIZE-T) studies for thalassemia along with P-II/III (RISE UP) study in SCD
Enjaymo
Active ingredient: sutimlimab Approved: November 17, 2022
Company: Sanofi Disease: Cold Agglutinin Disease
- The approval was based on the part A P-III trials (CADENZA) & (CARDINAL) evaluating Enjaymo (6.5/7.5g, IV on Day 0, Day 7, and then EOW for ~26wk.) vs PBO in 42 & 24 patients with CAD. The product will be available as a 50mg/mL solution for infusion
- Both the trial met its 1EPs & 2EPs i.e., In the (CADENZA) study, the therapy showed inhibition of hemolysis, an increase in Hb levels & improvement in FACIT-Fatigue scores along with an acceptable safety profile & well- tolerated, patients experienced 1 TEAE (96% vs 100%) without a recent history of blood transfusion
- In the (CARDINAL) study, the efficacy was evaluated based on the 1EPs & different 2EPs incl. improvements in Hb, normalization of bilirubin & FACIT-fatigue score who have had a recent blood transfusion
Brukinsa
Active ingredient: zanubrutinib Approved: November 18, 2022
Company: BeiGene Disease: Chronic Lymphocytic Leukemia
- The EC has approved Brukinsa in adult patients with TN or r/r CLL
- The approval was based on the P-III (SEQUOIA) and (ALPINE) trial evaluating zanubrutinib (BTK inhibitor) vs bendamustine + rituximab or ibrutinib in 740 & 652 which showed superior efficacy in both trials & superiority to chemoimmunotherapy, ORR (80.4% vs 72.9%), and patients had a sustained response @1yr. with rates of 90% vs 78%, favorable safety profile incl. lower rates of AF/flutter
- The therapy has been approved in the EU for WM in adult patients who have received 1 prior therapy or as a 1L treatment for patients unsuitable for chemoimmunotherapy & for MZL who have received 1 prior anti-CD20-based therapy
Libtayo
Active ingredient: cemiplimab Approved: November 23, 2022
Company: Regeneron Disease: Cervical Cancer
- The EC has approved Libtayo as monotx. in adult patients with recurrent or metastatic cervical cancer & disease progression on or after Pt-based CT
- The approval was based on the P-III (EMPOWER-Cervical 1) trial evaluating Libtayo (350mg, q3w) as monotx. vs CT in 608 patients across 14 countries irrespective of PD-L1 expression status or histology which showed an improvement in OS, PFS & ORR, 31% & 27% reduction in risk of death & a longer m-OS in the overall population & SCC histology (12.0 vs 8.5mos. & 11.1 vs 8.8mos.), 25% reduction of risk in progressive disease while ORR (16% vs 6%) in the overall population
- Immune-mediated adverse reactions (21%), permanent discontinuation (4.6%) with no new Libtayo safety signals. The results were published in NEJM
Skyrizi
Active ingredient: risankizumab Approved: November 23, 2022
Company: AbbVie Disease: Crohn’s Disease
- The approval was based on the data from the 3 P-III clinical studies (ADVANCE/MOTIVATE induction & FORTIFY maintenance) evaluating the safety, efficacy & tolerability of Skyrizi vs PBO as induction (600-1200mg) & maintenance (180-360mg) therapy in patients with moderately/severely active Crohn’s disease
- The patients treated with Skyrizi (600mg, IV) in the induction trials depicted a clinical remission of (43% & 35%) & an endoscopic response in (40% & 29%) @12wks vs (22% & 19%) & (12% & 11%) in PBO whereas those treated with Skyrizi (360mg, SC) in the maintenance trial depicted a clinical remission of 52% & an endoscopic response of 47% @52wks. vs 40% & 22% in PBO
- The induction trials showed mucosal healing & endoscopic remission in (21% & 14%) & (24% & 19%) of patients @12wks. vs (8% & 4%) & (9% & 4%) in PBO whereas for that in the maintenance trial the number was 31% & 39% vs 10% & 13% @52wks.
Biktarvy
Active ingredient: bictegravir Approved: November 29, 2022
Company: Gilead Disease: HIV
- The EC has authorized the new low-dose of Biktarvy (bictegravir 30mg/ emtricitabine 120mg/ tenofovir alafenamide 15mg) for HIV inf. in virologically suppressed children aged 2yrs. The authorization was based on the open-label study of Biktarvy
- The therapy was found to be effective & well-tolerated @24wks. In (Study 1474), treatment outcomes were evaluated in adolescents aged b/w 12-≤18yrs. (cohort 1), children b/w 6-≤12yrs. (cohort 2) & children aged 2yrs. (cohort 3)
- 98% in cohorts 1 & 2 remained suppressed @48wks. after switching to Biktarvy, a similar result was observed in cohort 3 & 91% remained virologically suppressed @24wks. with no new adverse reactions in pediatric patients aged ≥2yrs. The EC’s marketing authorization will be valid in all 27 member states of the EU, Norway, Iceland & Liechtenstein
Related Post: Insights+: EMA Marketing Authorization of New Drugs in October 2022




