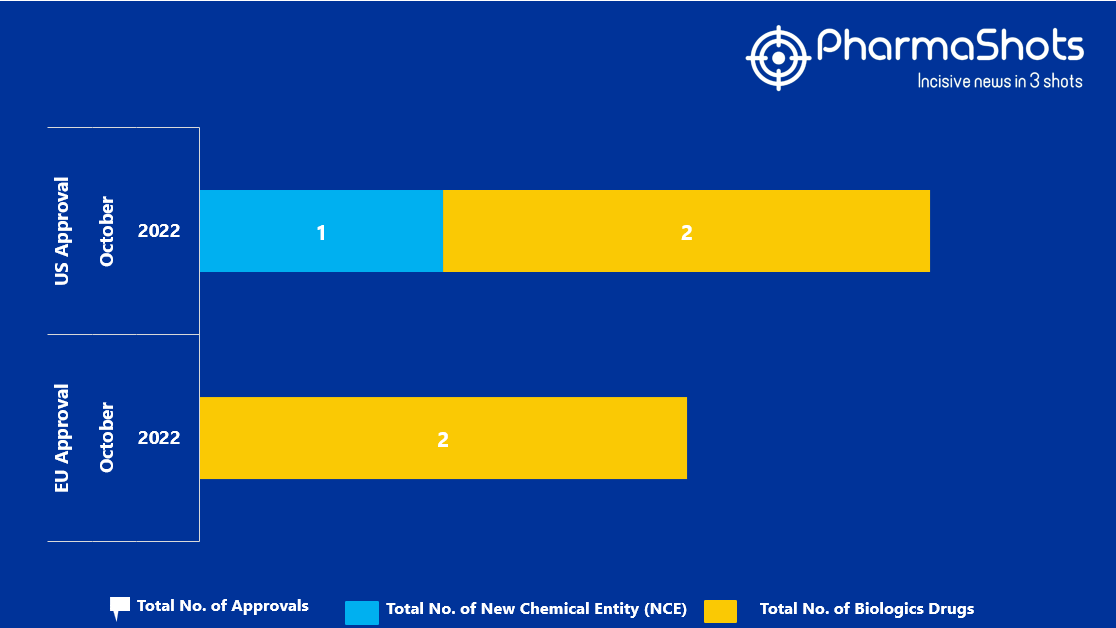
Insights+: EMA Marketing Authorization of New Drugs in October 2022
- The EMA approved 2 Biologic Drugs in October 2022, leading to treatments for patients and advances in the healthcare industry
- In October 2022, the major highlights drugs were Yescarta approval for diffuse large B-cell lymphoma and high-grade B-cell lymphoma, and Adtralza for atopic dermatitis
- PharmaShots has compiled a list of a total of 2 new drugs approved by the EMA in October 2022
Yescarta
Active ingredient: axicabtagene ciloleucel Approved: October 18, 2022
Company: Kite Pharma Disease: B-cell Lymphoma
- The EC has granted approval to Yescarta for adult patients with DLBCL and HGBL who relapse within 12mos. from completion or are refractory to 1L chemoimmunotherapy
- The approval was based on the P-III (ZUMA-7) study evaluating Yescarta vs SoC in 359 adult patients with r/r LBCL within 12mos. at 77 centers. The study showed a ≥4-fold improvement in 1EPs of EFS (8.3mos. vs 2.0mos.) at a median follow-up of 2yrs. & had a safety profile that was consistent with prior studies
- Additionally, a 2.5-fold increase in patients who were alive @2yrs. without disease progression or need for additional treatment (41% v 16%). The results were consistent in patient subgroups, grade ≥3 CRS & neurologic events (6% & 21%) with no grade 5 CRS or neurologic events
2. LEO’s Adtralza Receives EC’s Approval for the Treatment of Moderate to Severe Atopic Dermatitis
Adtralza
Active ingredient: tralokinumab Approved: October 21, 2022
Company: LEO Pharma Disease: Atopic Dermatitis
- The approval was based on the P-III (ECZTRA 6) trial evaluating the safety & efficacy of Adtralza monothx. (150/300mg) vs PBO in adolescents (n=289) aged 12-17yrs. with moderate-to-severe AD who were candidates for systemic therapy
- Post-washout period patients were given Adtralza (Q2W) or PBO initially for 16wks. & dosing of Adtralza started with 300 or 600mg on day 0 for patients receiving 150/300mg, Q2W. Patients responding to Adtralza @16wks. with an IGA score of 0/1 and/or 75% EASI change from baseline were randomized to Adtralza (Q2W/Q4W) for an additional 36wks.
- Adtralza is a high-affinity human mAb that binds to & inhibits the IL-13 cytokine responsible for the immune & inflammatory processes underlying AD, thereby inhibiting interaction with the IL-13 receptor α1 and α2 subunits
Related Post: Insights+: EMA Marketing Authorization of New Drugs in September 2022



